Products
Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
This kit is based on Double antibody-Sandwich ELISA detection method and takes 4h assay time. The microplate provided in this kit has been precoated with anti TNF-α antibody. Add standard and properly diluted sample into relevant well respectively. After incubation, wash unbound components. Add biotinylated detection antibody. Then, it binds with TNF-α bound to precoated antibody. Wash unbound components and add HRP-Streptavidin Conjugate (SABC). Wash unbound components again and add TMB substrate solution. Then, TMB was catalyzed by HRP to produce a blue color product that turned yellow after adding a stop solution. Read the O.D. absorbance at 450nm in a microplate reader. Calculate the concentration of TNF-α in the sample by plotting standard curve. The concentration of the target substance is proportional to the OD450 value.
- Catalogue No.:
- ER1393
- Alias:
- Tumor necrosis factor ELISA Kit, Cachectin ELISA Kit, TNF-alpha ELISA Kit, Tumor necrosis factor ligand superfamily member 2 ELISA Kit, TNF-a ELISA Kit, Tumor necrosis factor, membrane form ELISA Kit, N-terminal fragment ELISA Kit, NTF ELISA Kit, Intracellular domain 1 ELISA Kit, ICD1 ELISA Kit, Intracellular domain 2 ELISA Kit, ICD2 ELISA Kit, C-domain 1 ELISA Kit, C-domain 2 ELISA Kit, Tumor necrosis factor, soluble form ELISA Kit, TNF ELISA Kit, TNFA ELISA Kit, TNFSF2 ELISA Kit
- Species:
- Rat
- Range:
- 3.906-250pg/ml
- Sensitivity:
- 2.344pg/ml
| Size | Price |
|---|---|
| 96T | Inquiry |
- SPECIFICATIONS
- CITATIONS
- FIGURES
- CONDITIONS
- FAQS
- Product Name
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Alias
- Tumor necrosis factor ELISA Kit, Cachectin ELISA Kit, TNF-alpha ELISA Kit, Tumor necrosis factor ligand superfamily member 2 ELISA Kit, TNF-a ELISA Kit, Tumor necrosis factor, membrane form ELISA Kit, N-terminal fragment ELISA Kit, NTF ELISA Kit, Intracellular domain 1 ELISA Kit, ICD1 ELISA Kit, Intracellular domain 2 ELISA Kit, ICD2 ELISA Kit, C-domain 1 ELISA Kit, C-domain 2 ELISA Kit, Tumor necrosis factor, soluble form ELISA Kit, TNF ELISA Kit, TNFA ELISA Kit, TNFSF2 ELISA Kit
- Catalogue No.
- ER1393
- Size
- 48T/96T
- Species
- Rat
- UniProt ID
- P16599
- Sample Type
- Serum, Plasma, Cell Culture Supernatant, cell or tissue lysate, Other liquid samples
- Detection Method
- Sandwich ELISA, Double Antibody
- Detection Wavelength
- OD450
- Reaction Duration
- 4 hours
- Range
- 3.906-250pg/ml
- Sensitivity
- 2.344pg/ml
- Storage
- 2-8°C(Sealed), Don't cryopreserve.
- Specificity
- Specifically binds with TNF-α , no obvious cross reaction with other analogues.
- Recommended Sample Dilution Ratio
-
The following table shows the recommended dilution ratios for this kit for a limited number of samples for your reference only. (The matrix components in serum/plasma will affect the test results, which it need to be diluted at least 1/2 with Sample Dilution Buffer before testing! When the content of other samples is very low, the original solution can be added without dilution, but it is necessary to ensure that the pH is between 6.8 and 8.0, and it does not contain more than 10% organic solvents or high-concentration protein denaturants.)
1. Common sample validation:
Sample Type Recommended Dilution Ratio Content Normal Rat serum (n=15) 1/2 dilution ND-25pg/ml Normal Rat plasma (EDTA, Citrate , heparin) (n=15) 1/2 dilution ND-30pg/ml Rat spleen cells were cultured with 5%FBS + 1640 + double antibody for 48 hours 1/2dilution 15pg/ml Rat spleen cells were cultured with 5%FBS + 1640 + double antibody + 100 ng/ml LPS for 48 hours 1/20 dilution 865pg/ml Rat spleen cells were cultured with 5%FBS+ 1640 + double antibody +100ng/ml LPS for 48 hours. After that, 300ng/ml Brefeldin A (BFA) was added and cultured for 3 hours. Add cell lysis buffer(Catalogue No.:E050), collect the lysate solution (total protein concentration measured by BCA assay: 2.1 mg/ml) to detect. 1/50 dilution 1.2ng/mg(total protein) 2. KD sample validation (Detect TNF-α-KD NR8383 cells):
Sample Type Dilution Ratio Content Wild NR8383 cells were stimulated with 1μg/ml LPS for 12 hours to detect cell culture supernatant 1/2-1/10 dilution 635pg/ml NR8383 cells (+siRNA) were stimulated with 1μg/ml LPS for 12 hours to detect cell culture supernatants 1/2 dilution ND NR8383 cells (+siRNA) were treated with 1μg/ml LPS for 12 hours and then cultured with 300ng/ml Brefeldin A (BFA) for 3 hours. Add cell lysis buffer(Catalogue No.:E050), collect the lysate solution (total protein concentration measured by BCA assay: 1.28 mg/ml) to detect. 1/2 dilution 10pg/mg(total protein) Note:ND is lower than the sensitivity of the kit and was not detected
3. 3. Antibody by WB KD validation (Detect TNF-α-KO NR8383 cells):
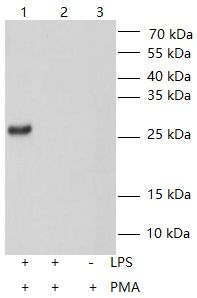 Lane 1: Wild NR8383 cells were treated with 1μg/ml LPS for 12 hours, then cultured with 300ng/ml Brefeldin A (BFA) for 3 hours. Add cell lysis buffer (Catalogue No.:E050), collect the lysate solution (total protein concentration measured by BCA assay: 1.58 mg/ml with 10ug total protein loading) to detect.
Lane 1: Wild NR8383 cells were treated with 1μg/ml LPS for 12 hours, then cultured with 300ng/ml Brefeldin A (BFA) for 3 hours. Add cell lysis buffer (Catalogue No.:E050), collect the lysate solution (total protein concentration measured by BCA assay: 1.58 mg/ml with 10ug total protein loading) to detect.
Lane 2: NR8383 cells (+siRNA) were treated with 1μg/ml LPS for 12 hours, then cultured with 300ng/ml Brefeldin A (BFA) for 3 hours. Add cell lysis buffer (Catalogue No.:E050), collect the lysate solution (total protein concentration measured by BCA assay: 2.05 mg/ml with 10ug total protein loading) to detect. (no obvious band)
Lane 3.Wild NR8383 cells were cultured for 12 hours (unstimulated), then cultured with 300 ng/ml Brefeldin A (BFA) for 3 hours. Add cell lysis buffer (Catalogue No.:E050), collect the lysate solution (total protein concentration measured by BCA assay: 1.33 mg/ml with 10ug total protein loading) to detect.(no obvious band)
Primary antibody:
All lanes :Capture mouse monoclonal antibody 1ug/ml
Secondary antibody:
All lanes : HRP-Goat Anti-mouse IgG (H+L) (Catalogue No.:FNSA-0003) at 1/5000 dilution
Molecular weight: 26 kDa
siRNA Target Sequences for Gene Knockdown:
Sense strand:5’-GUCCCAACAAGGAGGAGAA-3’
Antisense strand:5’-UUCUCCUCCUUGUUGGGAC-3’4. Verified recombinant protein:
(It is a normal phenomenon that some proteins have weak detection signals or cannot be detected at all due to differences in tags, sequences or protein activities)
A. HEK293-derived Rat TNF-alpha protein Leu80-Leu235
B. E.coli-derived Rat TNF-alpha protein Leu80-Leu235 - ELISA Kit Components
Kit Components Item Size(48T) Size(96T) Storage Condition for Opened Kit E001 ELISA Microplate(Dismountable) 8×6 8×12 Put the rest strips into a sealed foil bag with the desiccant. Stored for 1 month at 2-8°C; Stored for 6 month at -20°C E002 Lyophilized Standard 1vial 2vial Put the rest standards into a desiccant bag. Stored for 1 month at 2-8°C; Stored for 6 month at -20°C E003 Biotin-labeled Antibody(Concentrated, 100X) 60ul 120ul 2-8°C (Avoid Direct Light) E034 HRP-Streptavidin Conjugate(SABC, 100X) 60ul 120ul E024 TMB Substrate 5ml 10ml E039 Sample Dilution Buffer 10ml 20ml 2-8°C E040 Antibody Dilution Buffer 5ml 10ml E049 SABC Dilution Buffer 5ml 10ml E026 Stop Solution 5ml 10ml E038 Wash Buffer(Concentrated, 25X) 15ml 30ml E006 Plate Sealer 3 pieces 5 pieces E007 Product Description 1 copy 1 copy - Required Instruments and Reagents
-
- Microplate reader (wavelength: 450nm)
- 37°C incubator (CO2 incubator for cell culture is not recommenced.)
- Automated plate washer or multi-channel pipette/5ml pipettor (for manual washing purpose)
- Precision single (0.5-10μL, 5-50μL, 20-200μL, 200-1000μL) and multi-channel pipette with disposable tips(Calibration is required before use.)
- Sterile tubes and Eppendorf tubes with disposable tips
- Absorbent paper and loading slot
- Deionized or distilled water
- Assay Procedure Summary
-
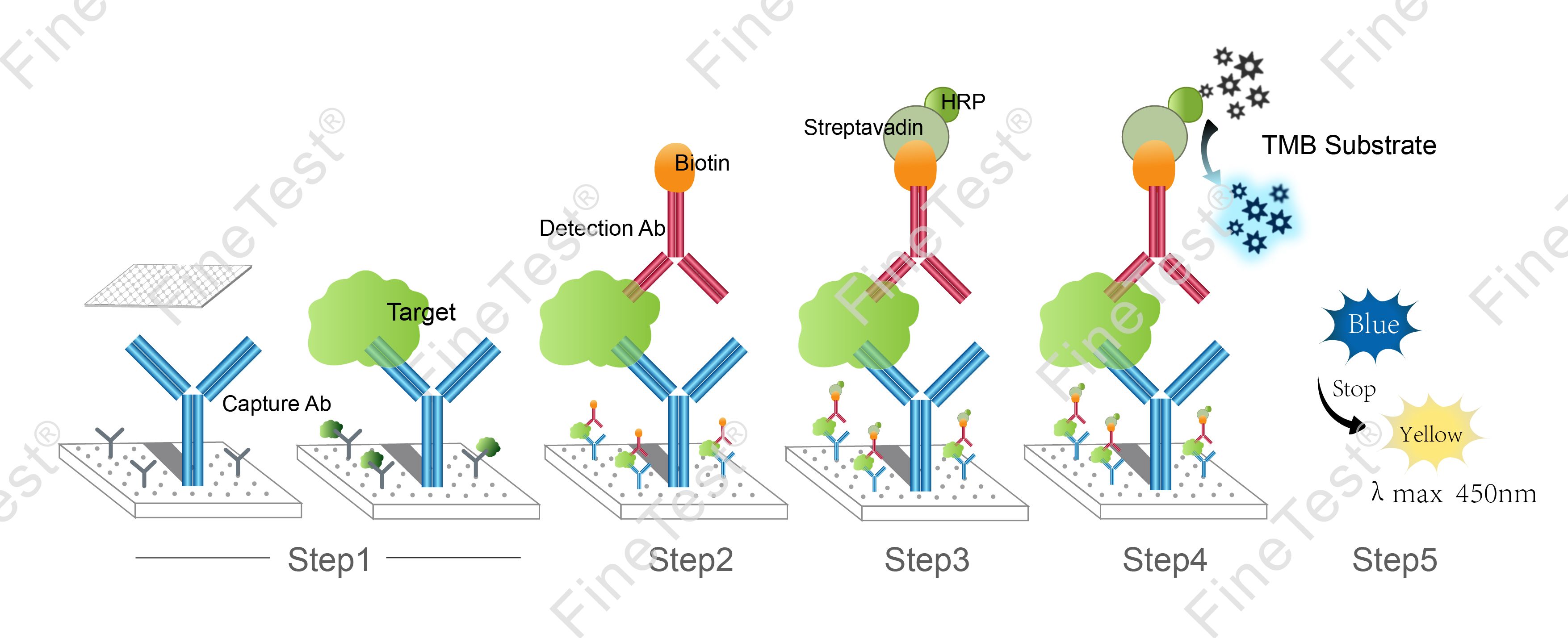
- Step 1: Add 100ul standard or sample into each well, seal the plate and statically incubate for 90 minutes at 37°C.
- Washing: Wash the plate twice without immersing.
- Step 2: Add 100ul biotin-antibody working solution, seal the plate and statically incubate for 60 minutes at 37°C.
- Washing: Wash the plate three times and immerse for 1min each time.
- Step 3: Add 100ul HRP-Streptavidin Conjugate (SABC) working solution, seal the plate and statically incubate for 30 minutes at 37°C.
- Washing: Wash the plate five times and immerse for 1min each time.
- Step 4: Add 90ul TMB substrate solution, seal the plate and statically incubate for 10-20 minutes at 37°C. (Accurate TMB visualization control is required.)
- Step 5: Add 50ul stop solution. Read at 450nm immediately and calculate.
- Standard Curve
-
This product is detected by QC department and meets performance required in the manual. (Laboratory Humidity: 20%-60%; Temperature: 18°C -25°C; Equilibrate TMB substrate to 37°C before staining. After adding into the ELISA wells, incubate for 15min at 37°C in dark.)
Due to different assay environments and operations, assay data below and standard curve are provided for reference. Experimenters should establish standard curve according to their own assay.
STD.(pg/ml) OD-1 OD-2 Average 0 0.088 0.092 0.09 3.906 0.111 0.116 0.113 7.813 0.134 0.14 0.137 15.625 0.264 0.274 0.269 31.25 0.433 0.45 0.442 62.5 0.742 0.772 0.757 125 1.242 1.292 1.267 250 2.005 2.087 2.046 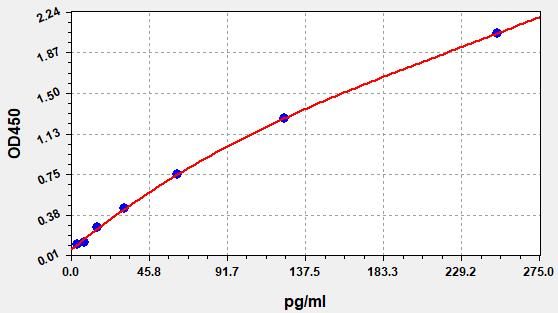
- Recovery
-
Add a certain amount of TNF-α into the sample. Calculate the recovery by comparing the measured value with the expected amount of TNF-α in the sample.
Sample Type Recovery Range(%) Average(%) serum(n=10) 88-96 93 EDTA plasma(n=10) 90-97 94 Heparin plasma(n=10) 86-104 96 - Linearity
-
Dilute the sample with a certain amount of TNF-α at 1:2, 1:4 and 1:8 to get the recovery range.
Sample Type 1:2 1:4 1:8 serum(n=10) 89-93% 86-105% 87-100% EDTA plasma(n=10) 85-98% 82-96% 83-101% Heparin plasma(n=10) 81-100% 81-99% 84-100% - Precision(%)
-
Intra-assay Precision: samples with low, medium and high concentration are tested 20 times on the same plate.
Inter-assay Precision: samples with low, medium and high concentration are tested 20 times on three different plates.
Item Intra-assay Precision Inter-assay Precision Sample 1 2 3 1 2 3 n 20 20 20 20 20 20 Mean (pg/ml) 7.78 30.39 124.29 8.11 30.97 125.2 Standard deviation 0.48 1.34 5.56 0.36 1.46 6 CV(%) 6.2 4.42 4.47 4.5 4.72 4.79 - Stability
-
Perform the stability test for the sealed kit at 37°C and 2-8°C and get relevant data.
ELISA kit(n=5) 37°C for 1 month 2-8°C for 6 months Average(%) 80 95-100
- Journal:
- Small
- Author:
- Institute of Functional Nano & Soft Materials (FUNSOM), Jiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Soochow University, 199 Ren'ai Road, Suzhou, Jiangsu, 215123, P. R. China.
- Cited Date:
- 2025-05-09
- Product:
- Journal:
- Advanced Healthcare Materials
- Author:
- Department of Ultrasound, Affiliated Hospital of North Sichuan Medical College, Nanchong, 637000, China.
- Sample:
- supernatant
- Cited Date:
- 2025-06-13
- Product:
- Journal:
- ACS Applied Materials & Interfaces
- Author:
- Department of Burn, Wound Repair & Reconstruction, The First Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong 510080, China
- Cited Date:
- 2023-08-11
- Product:
- Journal:
- Phytomedicine
- Author:
- Department of Orthopaedics and Traumatology, Affiliated Hospital of Nanjing University of Chinese Medicine, Jiangsu Provincial Hospital of Traditional Chinese Medicine, Nanjing 210023, China.
- Cited Date:
- 2025-11-28
- Product:
- Journal:
- Phytomedicine
- Author:
- Department of Traditional Chinese medicine, Inner Mongolia Medical University, Hohhot 010110, Inner Mongolia, China.
- Cited Date:
- 2025-10-11
- Product:
- Journal:
- Aging Cell
- Author:
- Department of Orthopedics, Renmin Hospital of Wuhan University, Wuhan, China.
- Sample:
- serum
- Cited Date:
- 2024-08-16
- Product:
- Journal:
- International Journal of Biological Macromolecules
- Author:
- South China Research Center for Acupuncture and Moxibustion, Guangzhou University of Chinese Medicine, Guangzhou 510006, Guangdong, PR China.
- Sample:
- supernatant
- Cited Date:
- 2024-10-08
- Product:
- Journal:
- Environmental Pollution
- Author:
- Henan Key Laboratory of Environmental and Animal Product Safety, College of Animal Science and Technology, Henan University of Science and Technology, Luoyang, 471000, Henan, China.
- Cited Date:
- 2025-12-12
- Product:
- Journal:
- JACC: Basic to Translational Science
- Author:
- Department of Critical Care Medicine, The First Affiliated Hospital of Xi'an Jiaotong University, Xi'an, Shaanxi, China.
- Cited Date:
- 2025-10-11
- Product:
- Journal:
- British Journal of Pharmacology
- Author:
- Hungarian Centre of Excellence for Molecular Medicine - University of Szeged, Cerebral Blood Flow and Metabolism Research Group, Szeged, Hungary.
- Sample:
- supernatants
- Cited Date:
- 2025-05-02
- Product:
- Journal:
- Phytomedicine
- Author:
- Southern Medical University Hospital of Integrated Traditional Chinese and Western Medicine, Peng Kang National Famous Traditional Chinese Medicine Expert Inheritance Studio, Southern Medicine University, Guangzhou 510315, China; Key Laboratory of Drug Me
- Cited Date:
- 2024-07-05
- Product:
- Journal:
- Biomedicine & Pharmacotherapy
- Cited Date:
- 2022-01-06
- Product:
- Journal:
- Molecular Medicine
- Cited Date:
- 2023-02-09
- Product:
- Journal:
- DRUG INVENTION TODAY
- Cited Date:
- 2019-08-25
- Product:
- Journal:
- Food & Function
- Cited Date:
- 2023-03-17
- Product:
- Journal:
- Ecotoxicology and Environmental Safety
- Author:
- The Key Laboratory of Environmental Pollution Monitoring and Disease Control, Ministry of Education, School of Public Health, Department of Toxicology, Guizhou Medical University,?Guian New Area, Guizhou 561113,?China.
- Sample:
- serum
- Cited Date:
- 2024-08-02
- Product:
- Journal:
- International Immunopharmacology
- Cited Date:
- 2023-04-14
- Product:
- Journal:
- International Immunopharmacology
- Author:
- Department of Intensive Care Unit, Baoding First Central Hospital, Baoding, China
- Cited Date:
- 2024-01-12
- Product:
- Journal:
- Journal of Functional Foods
- Author:
- Department of Pharmacognosy and Medicinal Plants, Faculty of Pharmacy, Future University in Egypt, 11835 Cairo, Egypt
- Cited Date:
- 2023-11-24
- Product:
- Journal:
- Industrial Crops and Products
- Author:
- Regional Plant Resource Centre, Medicinal & Aromatic Plant Division, Forest, Environment & Climate Change Department, Govt. of Odisha, Nayapalli, Bhubaneswar 751015, India
- Cited Date:
- 2024-08-23
- Product:
- Journal:
- Cellular Physiology and Biochemistry
- Cited Date:
- 2018-11-28
- Product:
- Journal:
- Food & Function
- Author:
- Department of Herbology, College of Korean Medicine, Dongguk University, Gyeongju 38066, Republic of Korea.
- Cited Date:
- 2025-09-26
- Product:
- Journal:
- Journal of Ethnopharmacology
- Author:
- Dongzhimen Hospital, Beijing University of Chinese Medicine, Beijing, 100700, China.
- Cited Date:
- 2025-10-24
- Product:
- Journal:
- Journal of Ethnopharmacology
- Author:
- School of Pharmacy, Hebei University of Chinese Medicine, Shijiazhuang, 050020, China.
- Cited Date:
- 2025-10-11
- Product:
- Journal:
- Journal of Ethnopharmacology
- Author:
- Regional Plant Resource Centre, Medicinal & Aromatic Plant Division, Forest & Environment Department, Govt. of Odisha, Nayapalli, Bhubaneswar, 751015, India
- Cited Date:
- 2023-07-28
- Product:
- Journal:
- Phytomedicine Plus
- Cited Date:
- 2021-09-02
- Product:
- Journal:
- Life Sciences
- Author:
- Department of Pharmacology and Biochemistry, Faculty of Pharmacy, Delta University for Science and Technology, International Coastal Road, Gamasa, Dakahliya, Egypt.
- Cited Date:
- 2024-10-18
- Product:
- Journal:
- Journal of Ethnopharmacology
- Cited Date:
- 2023-04-14
- Product:
- Journal:
- International Immunopharmacology
- Author:
- College of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing 100029, China.
- Cited Date:
- 2025-10-17
- Product:
- Journal:
- Experimental Neurology
- Author:
- The Third Central Clinical College of Tianjin Medical University, Tianjin 300170, China.
- Cited Date:
- 2025-06-06
- Product:
- Journal:
- Scientific Reports
- Author:
- Department of Animal Physiology and Ethology, Faculty of Natural Sciences, Comenius University, Ilkovicova 6, 842 15, Bratislava, Slovakia
- Cited Date:
- 2023-09-08
- Product:
- Journal:
- Toxics
- Author:
- Guangdong Provincial Key Laboratory of Utilization and Conservation of Food and Medicinal Resources in Northern Region, College of Biology and Agriculture, Shaoguan University, Shaoguan 512005, China.
- Species:
- testicular and epididymal tissue samples
- Cited Date:
- 2024-06-14
- Product:
- Journal:
- Biomedicine & Pharmacotherapy
- Cited Date:
- 2020-08-23
- Product:
- Journal:
- Molecular Nutrition & Food Research
- Author:
- Universitat Rovira i Virgili, Departament de Bioquímica i Biotecnologia, Nutrigenomics Research Group, C/ Marcel.li Domingo 1, Tarragona, 43007, Spain.
- Sample:
- serum
- Cited Date:
- 2025-06-06
- Product:
- Journal:
- Neurochemical Research
- Author:
- Department of Anesthesiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, 530021, China.
- Sample:
- serum and hippocampus
- Cited Date:
- 2024-03-22
- Product:
- Journal:
- Journal of Ethnopharmacology
- Cited Date:
- 2021-08-26
- Product:
- Journal:
- Journal of Ethnopharmacology
- Cited Date:
- 2022-03-24
- Product:
- Journal:
- Journal of Cellular and Molecular Medicine
- Cited Date:
- 2019-05-29
- Product:
- Journal:
- Experimental Gerontology
- Author:
- Department of Physiology and Biochemistry, Faculty of Medicine, Jordan University of Science and Technology, Irbid, 22110, Jordan.
- Cited Date:
- 2025-11-07
- Product:
- Journal:
- Pharmaceuticals
- Author:
- Department of Anatomy, Faculty of Medicine, Universiti Kebangsaan Malaysia, Jalan Yaacob Latif, Bandar Tun Razak, Kuala Lumpur 56000, Malaysia
- Sample:
- serum
- Cited Date:
- 2024-11-08
- Product:
-
- Rat INS(Insulin) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat PPAR-γ(Peroxisome ProlifeRator-Activated Receptor γ) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat HSD11B1(Corticosteroid 11-beta-dehydrogenase isozyme 1) ELISA Kit
- Rat CORT(Corticosterone) ELISA Kit
- Rat Lep(Leptin) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat ADP/Acrp30(Adiponectin) ELISA Kit
- Journal:
- Nutrients
- Cited Date:
- 2019-09-11
- Product:
- Journal:
- Brain Research Bulletin
- Cited Date:
- 2021-11-25
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat GROα/CXCL1(Growth Regulated Oncogene Alpha) ELISA Kit
- Rat IL-10(Interleukin-10) ELISA Kit
- Rat IL-17(Interleukin-17) ELISA Kit
- Rat IL-4(Interleukin-4) ELISA Kit
- Rat MIP-1α/CCL3(Macrophage Inflammatory Protein 1 Alpha) ELISA Kit
- Journal:
- Heliyon
- Author:
- The First Clinical Medical College, Guangxi Medical University, Guangxi, China
- Sample:
- serum
- Cited Date:
- 2024-04-19
- Product:
- Journal:
- BMC Microbiology
- Author:
- Department of Anesthesiology, The Affiliated Hospital of North Sichuan Medical College, 234 Fujiang Road, Shunqing District, Nanchong, Sichuan, 637000, China.
- Sample:
- spinal cord tissue
- Cited Date:
- 2025-05-16
- Product:
- Journal:
- Journal of Functional Foods
- Author:
- Hebei Key Laboratory of Integrative Medicine on Liver-kidney Patterns, Hebei University of Chinese Medicine, Shijiazhuang 050200, Hebei, China
- Cited Date:
- 2025-10-17
- Product:
- Journal:
- Green Processing and Synthesis
- Cited Date:
- 2023-03-17
- Product:
- Journal:
- Journal of Drug Targeting
- Author:
- Department of Biological Sciences, Sunandan Divatia School of Science, SVKM's NMIMS Deemed-to-be-University, Mumbai, Maharashtra, India.
- Cited Date:
- 2025-11-07
- Product:
- Journal:
- Biological Trace Element Research
- Author:
- The Key Laboratory of Environmental Pollution Monitoring and Disease Control, Ministry of Education, Department of Toxicology, School of Public Health, Guizhou Medical University, Guiyang, 550025, China
- Cited Date:
- 2023-12-22
- Product:
- Journal:
- Scientific Reports
- Author:
- Department of Neurosurgery, Neuromedicine Center, Beijing Shijitan Hospital, Capital Medical University, No. 10, Tieyi Road, Yangfangdian, Haidian District, Beijing, 100038, China.
- Sample:
- serum
- Cited Date:
- 2024-12-13
- Product:
- Journal:
- Archives of Medical Science
- Author:
- Department of Rehabilitation Medicine, The Second Affiliated Hospital of Xi’an Medical University, Xi’an, Shaanxi, China
- Cited Date:
- 2023-08-18
- Product:
-
- Rat CXCR2(C-X-C chemokine receptor type 2) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat SNCa(Synuclein Alpha) ELISA Kit
- Rat Th(Tyrosine Hydroxylase) ELISA Kit
- Rat Gfap(Glial fibrillary acidic protein) ELISA Kit
- Rat IL-17(Interleukin-17) ELISA Kit
- Rat IL-2(Interleukin-2) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Journal:
- Journal of Dental Sciences
- Cited Date:
- 2023-03-31
- Product:
- Journal:
- ACS Omega
- Author:
- Nutrition and Food Sciences Department, National Research Centre, Dokki, Giza 12622, Egypt
- Sample:
- blood sample
- Cited Date:
- 2024-07-12
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat LH(Luteinizing Hormone) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat FSH(Follicle Stimulating Hormone) ELISA Kit
- Rat Gc(Glucagon) ELISA Kit
- Rat Iapp(Islet amyloid polypeptide) ELISA Kit
- Rat Pth(PaRathyroid hormone) ELISA Kit
- Rat Lep(Leptin) ELISA Kit
- Rat IL-10(Interleukin-10) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Journal:
- Regenerative Therapy
- Cited Date:
- 2022-09-22
- Product:
- Journal:
- Naunyn-Schmiedeberg's Archives of Pharmacology
- Author:
- Department of Biochemistry, State University of Maringá, Paraná, Brazil
- Cited Date:
- 2023-07-07
- Product:
- Journal:
- Journal of Biochemical and Molecular Toxicology
- Cited Date:
- 2022-10-13
- Product:
- Journal:
- Journal of Biochemical and Molecular Toxicology
- Author:
- Department of Chemistry and Chemical Processing Technologies, Macka Vocational School Karadeniz Technical University Trabzon Turkiye
- Cited Date:
- 2023-06-30
- Product:
- Journal:
- Brain Research Bulletin
- Author:
- Department of Anesthesiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, China.
- Sample:
- serum
- Cited Date:
- 2024-09-27
- Product:
- Journal:
- Food and Chemical Toxicology
- Author:
- NITTE (Deemed to be University), Central Research Laboratory, K. S. Hegde Medical Academy, Deralakatte, Mangalore, 575018, Karnataka, India.
- Cited Date:
- 2026-02-13
- Product:
- Journal:
- Psychopharmacology
- Author:
- Department of Pharmacology and Toxicology, Faculty of Pharmacy, Ain Shams University, Cairo, Egypt.
- Sample:
- brain homogenates
- Cited Date:
- 2025-01-24
- Product:
- Journal:
- Nutrition & Metabolism
- Cited Date:
- 2019-02-27
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat NF-κB p65(Nuclear Factor-κB P65) ELISA Kit
- Rat MAPK(P38 Mitogen-Activated Protein Kinase) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat Nfe2l2(Nuclear Factor, Erythroid Derived 2 Like Protein 2) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat MCP-1(Monocyte Chemotactic Protein 1) ELISA Kit
- Journal:
- Journal of Ethnopharmacology
- Cited Date:
- 2020-06-11
- Product:
- Journal:
- AAPS PharmSciTech
- Author:
- Department of Pharmaceutics, Faculty of Pharmacy, Mansoura University, Mansoura, 35516, Dakahlia, Egypt.
- Sample:
- lung homogenate
- Cited Date:
- 2025-01-24
- Product:
- Journal:
- Reproductive Toxicology
- Author:
- Department of Medical Services and Techniques, Vocational School of Health Services,?Karadeniz Technical University,?Trabzon 61080,?Turkiye.
- Cited Date:
- 2024-07-19
- Product:
-
- Rat ATF6(Activating transcription factor 6) ELISA Kit
- Rat NQO1(NAD(P)H dehydrogenase [quinone] 1) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat NF-κB p65(Nuclear Factor-κB P65) ELISA Kit
- Rat HO-1(Heme Oxygenase 1) ELISA Kit
- Rat BCL-2(B-Cell Leukemia/Lymphoma 2) ELISA Kit
- Rat Ddit3(DNA damage-inducible transcript 3 protein) ELISA Kit
- Rat Nfe2l2(Nuclear Factor, Erythroid Derived 2 Like Protein 2) ELISA Kit
- Rat Hspa5(78 kDa glucose-regulated protein) ELISA Kit
- Rat Bax(Apoptosis regulator BAX) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Rat Gpx1(Glutathione peroxidase 1) ELISA Kit
- Rat Hmgb1(High mobility group protein B1) ELISA Kit
- Rat MPO(Myeloperoxidase) ELISA Kit
- Rat Casp3(Caspase 3) ELISA Kit
- Journal:
- Reproductive Medicine and Biology
- Author:
- Department of Biology, Ur.C Islamic Azad University Urmia Iran.
- Cited Date:
- 2025-10-31
- Product:
- Journal:
- Food & Function
- Cited Date:
- 2020-07-30
- Product:
- Journal:
- Molecular Pain
- Cited Date:
- 2019-02-05
- Product:
- Journal:
- Molecular Pain
- Cited Date:
- 2019-05-30
- Product:
- Journal:
- Journal of Biochemical and Molecular Toxicology
- Author:
- Department of Animal Physiology, Damghan Branch, Islamic Azad University, Damghan, Iran.
- Sample:
- serum
- Cited Date:
- 2024-09-13
- Product:
-
- Rat MDA(Malonaldehyde) ELISA Kit
- Rat 8-OHdG(8-Hydroxydeoxyguanosine) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat hs-CRP(High Sensitivity C-Reactive Protein) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Rat Gpx1(Glutathione peroxidase 1) ELISA Kit
- Rat Cat(Catalase) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Journal:
- Naunyn-Schmiedeberg's Archives of Pharmacology
- Author:
- Faculty of Veterinary Medicine, Department of Physiology, Hatay Mustafa Kemal University, Hatay, Turkey.
- Cited Date:
- 2024-11-15
- Product:
- Journal:
- The Kaohsiung Journal of Medical Sciences
- Author:
- Department of Nephrology, China Resources & WISCO General Hospital, Affiliated to Wuhan University of Science and Technology, Wuhan, Hubei, China.
- Cited Date:
- 2025-12-05
- Product:
- Journal:
- Naunyn-Schmiedeberg's Archives of Pharmacology
- Author:
- Chemistry Department, Faculty of Science, Damietta University, Damietta, 34517, Egypt.
- Cited Date:
- 2025-11-14
- Product:
- Journal:
- Current Alzheimer Research
- Cited Date:
- 2020-09-05
- Product:
- Journal:
- Applied Biochemistry and Biotechnology
- Author:
- Zhucheng Maternal and Child Health Center, No. 343 Dongguan Street, Zhucheng, Weifang, Shandong, 262200, People’s Republic of China
- Cited Date:
- 2023-07-21
- Product:
- Journal:
- Current Issues in Molecular Biology
- Author:
- Department of Physiology Guangxi Medical University, Nanning 530021, China
- Cited Date:
- 2025-10-17
- Product:
- Journal:
- Human and Experimental Toxicology
- Cited Date:
- 2021-07-09
- Product:
-
- Rat CXCR4(C-X-C motif receptor 4) ELISA Kit
- Rat TGF-β1(Transforming Growth Factor Beta 1) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat IL-21(Interleukin-21) ELISA Kit
- Rat MMP-9(Matrix Metalloproteinase-9) ELISA Kit
- Rat TIMP-1(Tissue Inhibitors of Metalloproteinase 1) ELISA Kit
- Rat TIMP-2(Tissue Inhibitors of Metalloproteinase 2) ELISA Kit
- Rat IL-10(Interleukin-10) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat MMP-2(Matrix Metalloproteinase 2) ELISA Kit
- Rat IFN-γ(Interferon γ) ELISA Kit
- Journal:
- Immunological Investigations
- Author:
- Obstetrics Department, Zibo Central Hospital, Zibo, Shandong, China.
- Cited Date:
- 2024-11-15
- Product:
- Journal:
- Obesity Surgery
- Author:
- Department of Gastroenterology, Affiliated Hospital of North Sichuan Medical College, South Maoyuan Road, Shunqing District, Nanchong City, 637000, Sichuan Province, China
- Cited Date:
- 2024-03-01
- Product:
- Journal:
- Brain Research
- Author:
- Obstetrics Department, Maternal and Child Health Hospital of Hubei Province, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430070, P.R. China
- Cited Date:
- 2023-09-01
- Product:
- Journal:
- Journal of Pharmacy and Pharmacology
- Author:
- Department of Pharmacology, School of Pharmaceutical Education and Research, Jamia Hamdard, Hamdard, New Delhi 110062, India.
- Cited Date:
- 2024-09-13
- Product:
- Journal:
- Molecular Biology Reports
- Author:
- Department of Biological Sciences, Faculty of Science, Yarmouk University, Irbid, 211-63, Jordan.
- Cited Date:
- 2025-08-01
- Product:
- Journal:
- Cardiovascular Toxicology
- Cited Date:
- 2023-04-14
- Product:
- Journal:
- Journal of Food Biochemistry
- Cited Date:
- 2021-12-30
- Product:
- Journal:
- South African Journal of Botany
- Author:
- Department of Chemistry and Chemical Processing Technologies, Macka Vocational School, Karadeniz Technical University, 61750 Trabzon, Turkiye
- Cited Date:
- 2025-02-14
- Product:
-
- Rat ATF6(Activating transcription factor 6) ELISA Kit
- Rat NQO1(NAD(P)H dehydrogenase [quinone] 1) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat NF-κB p65(Nuclear Factor-κB P65) ELISA Kit
- Rat HO-1(Heme Oxygenase 1) ELISA Kit
- Rat G6PD(Glucose 6 Phosphate Dehydrogenase) ELISA Kit
- Rat Ddit3(DNA damage-inducible transcript 3 protein) ELISA Kit
- Rat Nfe2l2(Nuclear Factor, Erythroid Derived 2 Like Protein 2) ELISA Kit
- Rat Hspa5(78 kDa glucose-regulated protein) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Rat Gpx1(Glutathione peroxidase 1) ELISA Kit
- Rat Hmgb1(High mobility group protein B1) ELISA Kit
- Rat MPO(Myeloperoxidase) ELISA Kit
- Rat Casp3(Caspase 3) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Journal:
- Pharmaceutical Development and Technology
- Author:
- Department of Pharmacology and Toxicology, Faculty of Pharmacy, Zagazig University, Zagazig, 44519, Egypt.
- Cited Date:
- 2024-09-27
- Product:
- Journal:
- International Journal of Clinical Practice
- Cited Date:
- 2021-11-11
- Product:
- Journal:
- Journal of Pain Research
- Author:
- Institute of Basic Medicine and Forensic Medicine, North Sichuan Medical College, Nanchong, Sichuan, People’s Republic of China
- Sample:
- supernatants
- Cited Date:
- 2024-07-26
- Product:
- Journal:
- Neurosurgical Review
- Author:
- Department of Infection Control, The?Affiliated Taizhou People's Hospital of Nanjing Medical University, Taizhou, Jiangsu Province, 225300, China.
- Cited Date:
- 2025-02-07
- Product:
- Journal:
- Current Pharmaceutical Design
- Cited Date:
- 2019-11-19
- Product:
- Journal:
- Medicina
- Author:
- Department of Physiology, Faculty of Medicine, Kutahya Health Sciences University, Kutahya 43020, Turkey
- Sample:
- supernatants
- Cited Date:
- 2025-03-14
- Product:
- Journal:
- Respiratory Physiology & Neurobiology
- Author:
- Biomedical Centre Martin, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Mala hora 4C, 036 01 Martin, Slovakia
- Cited Date:
- 2023-08-18
- Product:
- Journal:
- Pulmonary Circulation
- Cited Date:
- 2019-08-02
- Product:
- Journal:
- Pulmonary Circulation
- Cited Date:
- 2020-04-10
- Product:
- Journal:
- Pulmonary Circulation
- Cited Date:
- 2019-06-25
- Product:
- Journal:
- Archives of Physiology and Biochemistry
- Cited Date:
- 2020-05-16
- Product:
- Journal:
- European Journal of Trauma and Emergency Surgery
- Author:
- Department of Chemistry and Chemical Processing Technologies, Macka Vocational School, Karadeniz Technical University, 61750, Trabzon, Turkey
- Cited Date:
- 2023-07-14
- Product:
-
- Rat ATF6(Activating transcription factor 6) ELISA Kit
- Rat 8-OHdG(8-Hydroxydeoxyguanosine) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat NF-κB p65(Nuclear Factor-κB P65) ELISA Kit
- Rat Ddit3(DNA damage-inducible transcript 3 protein) ELISA Kit
- Rat Hspa5(78 kDa glucose-regulated protein) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Rat Hmgb1(High mobility group protein B1) ELISA Kit
- Rat Cat(Catalase) ELISA Kit
- Rat MPO(Myeloperoxidase) ELISA Kit
- Rat Casp3(Caspase 3) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Journal:
- Nutrition and Cancer
- Cited Date:
- 2020-03-28
- Product:
- Journal:
- Medical Science Monitor
- Cited Date:
- 2020-01-26
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat NAGase(N-Acetyl Beta-D-Glucosaminidase) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat CST3 (Cystatin C) ELISA Kit
- Rat BMG/β2-MG(Beta-2-Microglobulin) ELISA Kit
- Rat IL-17(Interleukin-17) ELISA Kit
- Rat IL-18(Interleukin-18) ELISA Kit
- Rat IL-2(Interleukin-2) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat Kim-1(Kidney Injury Molecule 1) ELISA Kit
- Rat Lipocalin-2/NGAL(Neutrophil Gelatinase Associated Lipocalin) ELISA Kit
- Journal:
- Alternative Therapies in Health and Medicine
- Author:
- School of Public Health, Xi’an Jiaotong University, Xi’an Shaanxi, China.
- Sample:
- blood samples
- Cited Date:
- 2024-08-02
- Product:
- Journal:
- Nutrition & Food Science
- Cited Date:
- 2021-09-29
- Product:
- Journal:
- Neuroimmunomodulation
- Cited Date:
- 2021-05-07
- Product:
- Journal:
- Biotechnology and Applied Biochemistry
- Cited Date:
- 2020-10-30
- Product:
- Journal:
- Pharmacology
- Cited Date:
- 2019-08-30
- Product:
- Journal:
- Cellular and Molecular Biology
- Author:
- Department of Traumatic Surgery& Emergency Surgery, The 1st Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang,325000, China
- Cited Date:
- 2023-10-13
- Product:
- Journal:
- Bratislava Medical Journal
- Author:
- Faculty of Medicine, Kutahya Health Sciences University, Kutahya, Turkey
- Sample:
- supernatants and plasma
- Cited Date:
- 2025-05-09
- Product:
- Journal:
- Journal of Nanoscience and Nanotechnology
- Cited Date:
- 2019-04-20
- Product:
- Journal:
- Neuroimmunomodulation
- Cited Date:
- 2020-06-10
- Product:
- Journal:
- The Journal of Basic and Applied Zoology
- Author:
- Zoology Department, Faculty of Science, Aswan University, Aswan, 81582, Egypt
- Sample:
- liver tissue
- Cited Date:
- 2025-08-22
- Product:
- Journal:
- The Journal of Basic and Applied Zoology
- Author:
- Department of Human Physiology, College of Medical Sciences, Faculty of Basic Medical Sciences, Ahmadu Bello University, Zaria, Kaduna, Nigeria
- Cited Date:
- 2025-12-26
- Product:
- Journal:
- Indian Journal of Experimental Biology
- Author:
- C U Shah College of Pharmacy, S.N.D.T. Women’s University, Juhu Campus, Juhu Rd, Santacruz (W), Mumbai - 400 049, Maharashtra, India
- Cited Date:
- 2023-06-09
- Product:
- Journal:
- The Eurasian Journal of Medicine
- Author:
- Department of Pharmaceutical Technology and Management, Azerbaijan Medical University Faculty of Pharmacy Baku, Azerbaijan
- Sample:
- supernatant
- Cited Date:
- 2024-11-01
- Product:
- Journal:
- Acta Poloniae Pharmaceutica - Drug Research
- Cited Date:
- 2020-03-13
- Product:
- Journal:
- Asian Pacific Journal of Tropical Medicine
- Cited Date:
- 2017-03-06
- Product:
- Journal:
- Revista Internacional de Andrología
- Author:
- Department of Nutrition and Dietetics, Faculty of Health Sciences, Karadeniz Technical University, 61080 Trabzon, Turkey
- Cited Date:
- 2024-05-06
- Product:
-
- Rat 8-OHdG(8-Hydroxydeoxyguanosine) ELISA Kit
- Rat ATF6(Activating transcription factor 6) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat NF-κB p65(Nuclear Factor-κB P65) ELISA Kit
- Rat Ddit3(DNA damage-inducible transcript 3 protein) ELISA Kit
- Rat Hspa5(78 kDa glucose-regulated protein) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Rat Hmgb1(High mobility group protein B1) ELISA Kit
- Rat Cat(Catalase) ELISA Kit
- Rat MPO(Myeloperoxidase) ELISA Kit
- Rat Casp3(Caspase 3) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Journal:
- Journal of Taibah University Medical Sciences
- Cited Date:
- 2019-07-23
- Product:
- Journal:
- European Journal of Inflammation
- Cited Date:
- 2018-05-14
- Product:
- Journal:
- Open Life Sciences
- Cited Date:
- 2021-02-26
- Product:
- Journal:
- Journal of Food and Nutrition Research
- Cited Date:
- 2018-12-19
- Product:
- Journal:
- Tropical Journal of Pharmaceutical Research
- Cited Date:
- 2021-10-08
- Product:
- Journal:
- Tropical Journal of Pharmaceutical Research
- Cited Date:
- 2022-06-17
- Product:
- Journal:
- Journal of Clinical Practice and Research
- Author:
- Department of Biophysics, Ayd?n Adnan Menderes University, Institute of Health Sciences, Ayd?n, Türkiye
- Sample:
- serum
- Cited Date:
- 2024-12-13
- Product:
-
- Rat INS(Insulin) ELISA Kit
- Rat MDA(Malonaldehyde) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat HbA1C(Glycosylated Hemoglobin/Hemoglobin A1c) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Rat Cat(Catalase) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Journal:
- Comparative Clinical Pathology
- Cited Date:
- 2019-06-25
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat pτ(phospho Tau Protein) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat CASP9(Caspase 9) ELISA Kit
- Rat Aβ40(Amyloid Beta 40) ELISA Kit
- Rat BCL-2(B-Cell Leukemia/Lymphoma 2) ELISA Kit
- Rat Bax(Apoptosis regulator BAX) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat BDNF(Brain Derived Neurotrophic Factor) ELISA Kit
- Journal:
- Tropical Journal of Natural Product Research
- Cited Date:
- 2021-02-26
- Product:
- Journal:
- Malaysian Journal of Medicine and Health Sciences (MJMHS)
- Cited Date:
- 2020-06-30
- Product:
- Journal:
- Research in Pharmaceutical Sciences
- Cited Date:
- 2022-09-08
- Product:
- Journal:
- HAYATI Journal of Biosciences
- Author:
- Benemerita Universidad Autonoma de Puebla, 3972, Centro de Agroecología, Instituto de Ciencias, Puebla, Puebla, Mexico.
- Sample:
- supernatant
- Cited Date:
- 2025-02-14
- Product:
- Journal:
- United States Patent Application
- Cited Date:
- 2020-07-16
- Product:
- Journal:
- Toxicology Research and Application
- Cited Date:
- 2021-03-25
- Product:
- Journal:
- Biointerface Research in Applied Chemistry
- Cited Date:
- 2023-02-09
- Product:
- Journal:
- Novosti Khirurgii
- Author:
- Department of Surgery and Transplantation, Belarusian State Medical University, Minsk, Dzerzhinsky Ave. 83, 220116, Republic of Belarus
- Cited Date:
- 2023-10-08
- Product:
- Journal:
- Environmental Analysis Health and Toxicology
- Author:
- Department of Human Anatomy, Kaduna State University, State, Nigeria
- Cited Date:
- 2024-01-19
- Product:
- Journal:
- International Journal of Stem Cells
- Cited Date:
- 2019-07-30
- Product:
-
- Rat TGF-β1(Transforming Growth Factor Beta 1) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat NF-κB(Nuclear Factor Kappa B) ELISA Kit
- Rat IL-1α(Interleukin 1 Alpha) ELISA Kit
- Rat Cxcl2 (C-X-C motif chemokine 2) ELISA Kit
- Rat Stat3(Signal transducer and activator of transcription 3) ELISA Kit
- Rat IL-10(Interleukin-10) ELISA Kit
- Rat IP-10/CXCL10(Interferon Gamma Induced Protein 10kDa) ELISA Kit
- Journal:
- International Journal of Cell and Biomedical Science
- Cited Date:
- 2023-02-10
- Product:
- Journal:
- Narra J
- Author:
- Department of Biochemistry, Faculty of Medicine, Universitas Islam Sultan Agung, Semarang, Indonesia.
- Sample:
- serum
- Cited Date:
- 2025-02-21
- Product:
- Journal:
- Indonesian Journal of Nutrition and Food
- Author:
- Department of Nutrition Sciences, Faculty of Medicine, Diponegoro University, Semarang 50275, Indonesia
- Sample:
- serum
- Cited Date:
- 2025-02-28
- Product:
- Journal:
- International Journal of Multidisciplinary Research and Analysis
- Author:
- Faculty of Medicine, Universitas Islam Sultan Agung, Jl Kaligawe KM 4 Semarang 50012
- Cited Date:
- 2024-05-06
- Product:
- Journal:
- Current Biomedicine
- Author:
- Division of Pharmacology and Toxicology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, West Java, Indonesia
- Cited Date:
- 2025-10-24
- Product:
- Journal:
- Clinical and Experimental Health Sciences
- Author:
- KARADENIZ TECHNICAL UNIVERSITY
- Cited Date:
- 2023-10-20
- Product:
- Journal:
- Research Square
- Cited Date:
- 2022-05-05
- Product:
-
- Rat MDA(Malonaldehyde) ELISA Kit
- Rat TGF-β1(Transforming Growth Factor Beta 1) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat Gsr(Glutathione reductase) ELISA Kit
- Rat PTGS2/COX-2(Prostaglandin G/H synthase 2) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Journal:
- AIP Conference Proceedings
- Cited Date:
- 2020-09-16
- Product:
- Journal:
- Veterinary Sciences and Practices
- Author:
- Hatay Mustafa Kemal University, Faculty of Veterinary Medicine, Department of Physiology, Hatay, Türkiye
- Sample:
- serum
- Cited Date:
- 2025-05-09
- Product:
- Journal:
- Frontiers in Health Informatics
- Author:
- Department of Physiology, Pharmacology and Biochemistry, College of Veterinary Medicine, University of Basrah, Iraq
- Sample:
- serum
- Cited Date:
- 2024-09-27
- Product:
- Journal:
- research square
- Cited Date:
- 2020-09-29
- Product:
-
- Rat PGE2(Prostaglandin E2) ELISA Kit
- Rat 8-iso-PGF2α(8-isoprostane) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat OVA sIgE(Ovalbumin specific Immunoglobulin E) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat CYSLTR2(Cysteinyl Leukotriene Receptor 2) ELISA Kit
- Rat IL-10(Interleukin-10) ELISA Kit
- Rat IL-13(Interleukin-13) ELISA Kit
- Rat IL-17(Interleukin-17) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Mouse Ltb4r(Leukotriene B4 receptor 1) ELISA Kit
- Journal:
- Preprints
- Author:
- Department of Biology, Faculty of Science, Gaziantep University, 27010, Gaziantep, Turkey
- Cited Date:
- 2026-01-16
- Product:
- Journal:
- Aswan University Journal of Environmental Studies
- Author:
- Zoology department, Faculty of Science, Aswan University, 81582 Aswan, Egypt.
- Cited Date:
- 2025-10-11
- Product:
- Journal:
- Bali Journal of Anesthesiology
- Cited Date:
- 2022-05-27
- Product:
- Journal:
- Research Square
- Author:
- Islamic Azad University
- Cited Date:
- 2024-02-18
- Product:
- Journal:
- SSRN
- Author:
- School of Basic Medical Sciences, Jiangxi Medical College, Nanchang University, Nanchang, Jiangxi 330006, P.R. China
- Sample:
- SCG and neuron culture medium
- Cited Date:
- 2024-07-26
- Product:
- Journal:
- Archives of Biological Sciences
- Author:
- Department of Physiology, College of Medicine, University of Bisha, Bisha, Saudi Arabia
- Cited Date:
- 2025-10-17
- Product:
- Journal:
- Journal of Applied Pharmaceutical Science
- Author:
- Division of Pharmacology and Toxicology, School of Veterinary Medicine and Biomedical Sciences, IPB University, Bogor, Indonesia
- Cited Date:
- 2025-10-17
- Product:
- Journal:
- Journal of Innovative Research in Life Sciences
- Author:
- Department of Biochemistry, Federal University Gusau, Zamfara State-Nigeria
- Sample:
- serum
- Cited Date:
- 2024-05-31
- Product:
- Journal:
- FUDMA Journal of Sciences
- Author:
- Department of Human Physiology, Faculty of Basic Medical Science, Ahmadu Bello University, Zaria, Nigeria.
- Sample:
- serum
- Cited Date:
- 2024-08-02
- Product:
- Journal:
- Comparative Clinical Pathology
- Cited Date:
- 2021-06-25
- Product:
- Journal:
- Archives of Biological Sciences
- Author:
- Department of Physiology, College of Medicine, University of Bisha, Bisha, Saudi Arabia
- Cited Date:
- 2025-10-17
- Product:
- Journal:
- Tropical Life Sciences Research
- Cited Date:
- 2022-11-18
- Product:
- Journal:
- Journal of Multidisciplinary Applied Natural Science
- Author:
- Universitas Sebelas Maret, Surakarta-57126 (Indonesia); Department of Pharmacy, Sekolah Tinggi Kesehatan Al-Fatah, Bengkulu-38223 (Indonesia)
- Cited Date:
- 2026-01-30
- Product:
- Journal:
- SSRN
- Author:
- Biotechnology Program, Basic and Applied Sciences Institute, Egypt-Japan University of Science and Technology (E-JUST), 21934, New Borg El-Arab City, Alexandria, Egypt
- Cited Date:
- 2024-03-01
- Product:
- Journal:
- Doklady Biological Sciences
- Author:
- Avtsyn Institute of Human Morphology, Petrovsky National Research Center of Surgery, Moscow, Russia.
- Cited Date:
- 2026-01-30
- Product:
-
- Rat MDA(Malonaldehyde) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat hs-CRP(High Sensitivity C-Reactive Protein) ELISA Kit
- Rat cTnI/TNNI3 (Cardiac Troponin I) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Rat IL-10(Interleukin-10) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Journal:
- Bilecik Seyh Edebali University Journal of Science
- Author:
- Department of Chemistry and Chemical Processing Technologies, Macka Vocational School, Karadeniz Technical University, 61750 Trabzon, Türkiye
- Sample:
- supernatants
- Cited Date:
- 2024-06-07
- Product:
- Journal:
- International Journal of Research in Pharmaceutical Sciences
- Cited Date:
- 2021-07-15
- Product:
- Journal:
- Sahel Journal of Life Sciences FUDMA
- Author:
- Department of Biochemistry and Molecular Biology, Federal University Dutsin-Ma, P.M.B 5001, Katsina State, Nigeria
- Sample:
- serum
- Cited Date:
- 2024-03-08
- Product:
- Journal:
- Journal of Nutrition and Health
- Cited Date:
- 2020-03-13
- Product:
- Journal:
- Journal of Advanced Veterinary Research
- Author:
- Department of Animal Product Technology, Faculty of Animal Science, Universitas Gadjah Mada, Jl. Fauna 3, Bulaksumur, Yogyakarta 55281, Indonesia.
- Sample:
- culture supernatants
- Cited Date:
- 2025-01-10
- Product:
- Journal:
- Research Square
- Cited Date:
- 2022-12-09
- Product:
- Journal:
- Tropical Journal of Natural Product Research
- Author:
- Biochemistry Department, Faculty of Medicine, Universitas Islam Sultan Agung, Semarang, Central Java, Indonesia
- Sample:
- blood samples
- Cited Date:
- 2025-06-13
- Product:
-
- Rat MDA(Malonaldehyde) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat NF-κB(Nuclear Factor Kappa B) ELISA Kit
- Rat Kl(Klotho) ELISA Kit
- Rat Gpx1(Glutathione peroxidase 1) ELISA Kit
- Rat Cat(Catalase) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat IGF-1(Insulin-Like Growth Factor 1) ELISA Kit
- Journal:
- Comparative Clinical Pathology
- Cited Date:
- 2019-02-15
- Product:
-
- 8-OHdG(8-Hydroxydeoxyguanosine) ELISA Kit
- MDA(Malondialdehyde) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Rat Gpx1(Glutathione peroxidase 1) ELISA Kit
- Rat Cat(Catalase) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Journal:
- F?rat University Medical Journal of Health Sciences
- Author:
- Gaziantep University, Faculty of Medicine, Department of Physiology, Gaziantep, T?RK?YE
- Cited Date:
- 2023-07-07
- Product:
- Journal:
- Phytomedicine Plus
- Author:
- Dairy Department, Food Industries and Nutrition Research Institute, National Research Centre, Cairo 12622, Egypt
- Species:
- blood samples
- Cited Date:
- 2024-06-14
- Product:
- Journal:
- Medical Laboratory Technology Journal
- Author:
- Department of Nutrition Science, Faculty of Medicine, Diponegoro University, Semarang, Central Java, Indonesia 50275
- Sample:
- blood samples
- Cited Date:
- 2025-06-20
- Product:
- Journal:
- Orthopaedics, Traumatology and Prosthetics
- Author:
- Uzhhorod National University. Ukraine
- Cited Date:
- 2025-11-14
- Product:
- Journal:
- Journal of International Dental and Medical Research
- Cited Date:
- 2020-05-02
- Product:
- Journal:
- Bulletin of Egyptian Society for Physiological Sciences
- Cited Date:
- 2022-03-10
- Product:
- Journal:
- HEPATITIS MONTHLY
- Cited Date:
- 2020-05-10
- Product:
-
- Rat MDA(Malonaldehyde) ELISA Kit
- Rat 8-OHdG(8-Hydroxydeoxyguanosine) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat CASP9(Caspase 9) ELISA Kit
- Rat BCL-2(B-Cell Leukemia/Lymphoma 2) ELISA Kit
- Rat Bax(Apoptosis regulator BAX) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Rat Gpx1(Glutathione peroxidase 1) ELISA Kit
- Rat Cat(Catalase) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Journal:
- Beni-Suef University Journal of Basic and Applied Sciences
- Cited Date:
- 2023-01-06
- Product:
-
- 8-OHdG(8-Hydroxydeoxyguanosine) ELISA Kit
- Rat ATF6(Activating transcription factor 6) ELISA Kit
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat NF-κB p65(Nuclear Factor-κB P65) ELISA Kit
- Rat Ddit3(DNA damage-inducible transcript 3 protein) ELISA Kit
- Rat Hspa5(78 kDa glucose-regulated protein) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Rat Hmgb1(High mobility group protein B1) ELISA Kit
- Rat Cat(Catalase) ELISA Kit
- Rat MPO(Myeloperoxidase) ELISA Kit
- Rat Casp3(Caspase 3) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Journal:
- Egyptian Pharmaceutical Journal
- Author:
- Drug Radiation Research Department, National Centre for Radiation Research and Technology, Egyptian Atomic Energy Authority (EAEA), Cairo, Egypt.
- Sample:
- heart homogenate
- Cited Date:
- 2025-04-18
- Product:
What’s the plate size in FineTest® ELISA Kits?
The ELISA plate follows the standard size of microplate: 127.64 mm x 85.60 mm x 14.22 mm(L x W x H).
How about the shelf life and stability of FineTest® ELISA Kits?
Valid for 12 months since the production date. For the shelf life of specific batch number, please check the label printed on the kit. Before delivery, all FineTest® ELISA Kits have been subject to strict quality test.
Which cloned antibodies for FineTest® ELISA Kits are used?
These information is proprietary. Please contact us to learn more about clonality (polyclonality or monoclonality) and host species.
Can I mix reagents from different batches of FineTest® ELISA Kits?
Not suggested. ELISA reagents are optimized for specific batch.
Can FineTest® ELISA Kits be used partially?
Yes. The ELISA plate is dismounted. Enough component volumes are offered by 96T ELISA kit, supporting two groups of standard curve.
How long can the diluted lyophilized standard be stored for continual use?
Used up within 12h.
Can standard curve be extended to any direction?
FineTest® can't support validation of standard concentration outside of standard curve. Ranges of standard curve have been validated among many batches and experimenters, showing stable and accurate performance. The lowest standard concentration is the minimized range for reliable detection results. Adding higher or lower concentration of standard may cause inconsistent signal or false positive.
Why does detection for serum/plasma sample by FineTest® ELISA Kits require for 1/2 dilution?
Matrix components in serum/plasma can affect detection results. Blocking components in sample dilution buffer can decrease or remove the interference. The dilution can reduce the matrix difference between sample and standard to get better accuracy.
What’s the half-life of protein in serum/plasma/cell culture supernatant?
FineTest® can't determine the half-life of protein in the sample(e.g. serum, plasma or cell culture supernatant). Usually, it's suggested to detect prepared sample immediately or aliquot sample to refrigerate in a disposable container. Avoid freeze-thaw cycle to prevent protein degradation.
What's the expected concentration for particularly analyzing my sample?
Due to the specificity of each sample, it's hard to forecast and depend on sample preparation as well as analytical characteristics. Please contact us to get detection data for reference.
How many samples can FineTest® ELISA Kits detect?
It depends on whether duplicate assay for your sample is required. Such as, you can detect 80 samples without duplication or 40 samples.
Why is sample loading conducted after equilibrating all kits’ components and samples to room temperature?
Temperature is the important factor for ELISA binding reaction. The reaction of samples at a consistent temperature requires for equilibrating all reagents to room temperature before the assay, including tested samples. Avoid the inaccurate ELISA assay results caused by temperature differences.
Why is duplicate assay suggested for ELISA assay?
To get more accurate assay results, it's strongly suggested to conduct duplicate assay for the standard and sample. Duplicate assay can calculate average value to ensure more accurate assay results and solve drift caused by misoperation during the assay. Calculation of CV evaluates experimental operation and precision of the ELISA kit.
How to get the standard curve with excellent linearity?
Follow the suggested method in the manual to store the standard. Before dissolving the standard, transient centrifugation is required to completely collect the powder. Confirm the fully dissolution and mixing of the standard(about 10min). Then, conduct steps of series dilution. Ensure the fully mixing and accurate pipetting in each step. Properly stop the staining. Choose suitable fitting equation to plot the standard curve.
Can ELISA plates be stacked together for incubation?
To keep the consistent environmental condition of all plates, stacking is not suggested during incubation.
Why is polypropylene tube used for standard dilution in some analyses?
Some proteins or analytes can bind with glass or polystyrene. However, they are not easy to bind with polypropylene tube.
When to stop ELISA reaction?
ELISA assay finally requires for enzyme-catalyzed substrate to complete the staining reaction. Stopping the reaction at the best time is the important factor for successful ELISA assay. When the blue complex appears after adding TMB substrate for 12min, read the O.D. absorbance at 620nm. The staining can be stopped, when the OD value of the darkest color at 620nm is between 0.8 - 0.9. The relevant OD value at 450nm for stopping the staining is between 2.0 - 2.5. If the OD value at 620nm is lower than 0.8, the staining duration can be properly extended.
Why is dual wavelength selected for reading in a microplate reader? What's the purpose of wavelength correction?
Dual wavelength for reading in ELISA assay mainly aims to remove non-specific background interference, and improve accuracy and reliability of the detection. FineTest® usually suggests to set the corrected wavelength as 570nm or 630nm in a microplate reader. The OD value may be higher when directly reading at 450nm. However, the accuracy is lower.
Why is 4 - pl curve fitting is required for generating standard curve?
FineTest® recommends to use ELISA curve fitting software and validate ELISA kit with 4 - pl curve fitting. 4 - pl curve fitting mainly aims to accurately describe the nonlinear dynamic characteristics of antigen - antibody binding. Thus, the quantitative accuracy of low or high concentration of sample is improved. Experimental errors are decreased. The reliability and reproducibility of assay results are ensured.
If sample OD value is higher than OD value of the highest point on the standard curve, what's the suggested solution?
Dilute the sample with dilution buffer and conduct the detection again. Ensure the detected value of the sample falls in the range of standard curve.
Are there any rewards to publish papers using FineTest® ELISA Kits?
If you published paper using FineTest® ELISA Kits, you will obtain US$150-US$550 coupon, please contact us for more details.