Products
Rat LH(Luteinizing Hormone) ELISA Kit
This kit is based on Competitive-ELISA detection method and takes 2h assay time. The microplate provided in this kit has been precoated with LH. LH in the sample or standard competes with a fixed amount of biotin-labeled LH for sites on a pre-coated antibody specific to LH. Free components are washed off. After adding HRP-Streptavidin Conjugate (SABC), biotin specifically binds with streptavidin to form immune complexes. Wash off unbound conjugate. Add TMB substrate solution. Then, TMB was catalyzed by HRP to produce a blue color product that turned yellow after adding a stop solution. Read the O.D. absorbance at 450nm in a microplate reader. Compare OD450 value of sample with the standard curve through curve fitting software to determine the concentration of LH in the sample. The concentration of the target substance is inversely proportional to the OD450 value.
- Catalogue No.:
- ER1123
- Alias:
- LH ELISA Kit, Luteinizing Hormone ELISA Kit, Lutropin ELISA Kit, Lutrophin ELISA Kit
- Species:
- Rat
- Range:
- 0.313-20mIU/ml
- Sensitivity:
- 0.188mIU/ml
| Size | Price |
|---|---|
| 96T | Inquiry |
- SPECIFICATIONS
- CITATIONS
- FIGURES
- CONDITIONS
- FAQS
- Product Name
- Rat LH(Luteinizing Hormone) ELISA Kit
- Alias
- LH ELISA Kit, Luteinizing Hormone ELISA Kit, Lutropin ELISA Kit, Lutrophin ELISA Kit
- Catalogue No.
- ER1123
- Size
- 48T/96T
- Species
- Rat
- UniProt ID
- P01230
- Sample Type
- Serum, Plasma, Cell Culture Supernatant, cell or tissue lysate, Other liquid samples
- Detection Method
- Competitive ELISA, Coated with Antigen
- Detection Wavelength
- OD450
- Reaction Duration
- 2 hours
- Range
- 0.313-20mIU/ml
- Sensitivity
- 0.188mIU/ml
- Storage
- 2-8°C(Sealed), Don't cryopreserve.
- Specificity
- Specifically binds with LH , no obvious cross reaction with other analogues.
- ELISA Kit Components
Kit Components Item Size(48T) Size(96T) Storage Condition for Opened Kit E001 ELISA Microplate(Dismountable) 8×6 8×12 Put the rest strips into a sealed foil bag with the desiccant. Stored for 1 month at 2-8°C; Stored for 6 month at -20°C E002 Lyophilized Standard 1vial 2vial Put the rest standards into a desiccant bag. Stored for 1 month at 2-8°C; Stored for 6 month at -20°C E003 Lyophilized Biotin-labeled Antibody(Concentrated) 1vial 1vial E034 HRP-Streptavidin Conjugate(SABC, 100X) 60ul 120ul 2-8°C (Avoid Direct Light) E024 TMB Substrate 5ml 10ml E005 Purified Water 200ul 200ul 2-8°C E039 Sample Dilution Buffer 10ml 20ml E040 Antibody Dilution Buffer 5ml 10ml E049 SABC Dilution Buffer 5ml 10ml E026 Stop Solution 5ml 10ml E038 Wash Buffer(Concentrated, 25X) 15ml 30ml E006 Plate Sealer 3 pieces 5 pieces E007 Product Description 1 copy 1 copy - Required Instruments and Reagents
-
- Microplate reader (wavelength: 450nm)
- 37°C incubator (CO2 incubator for cell culture is not recommenced.)
- Automated plate washer or multi-channel pipette/5ml pipettor (for manual washing purpose)
- Precision single (0.5-10μL, 5-50μL, 20-200μL, 200-1000μL) and multi-channel pipette with disposable tips(Calibration is required before use.)
- Sterile tubes and Eppendorf tubes with disposable tips
- Absorbent paper and loading slot
- Deionized or distilled water
- Assay Procedure Summary
-
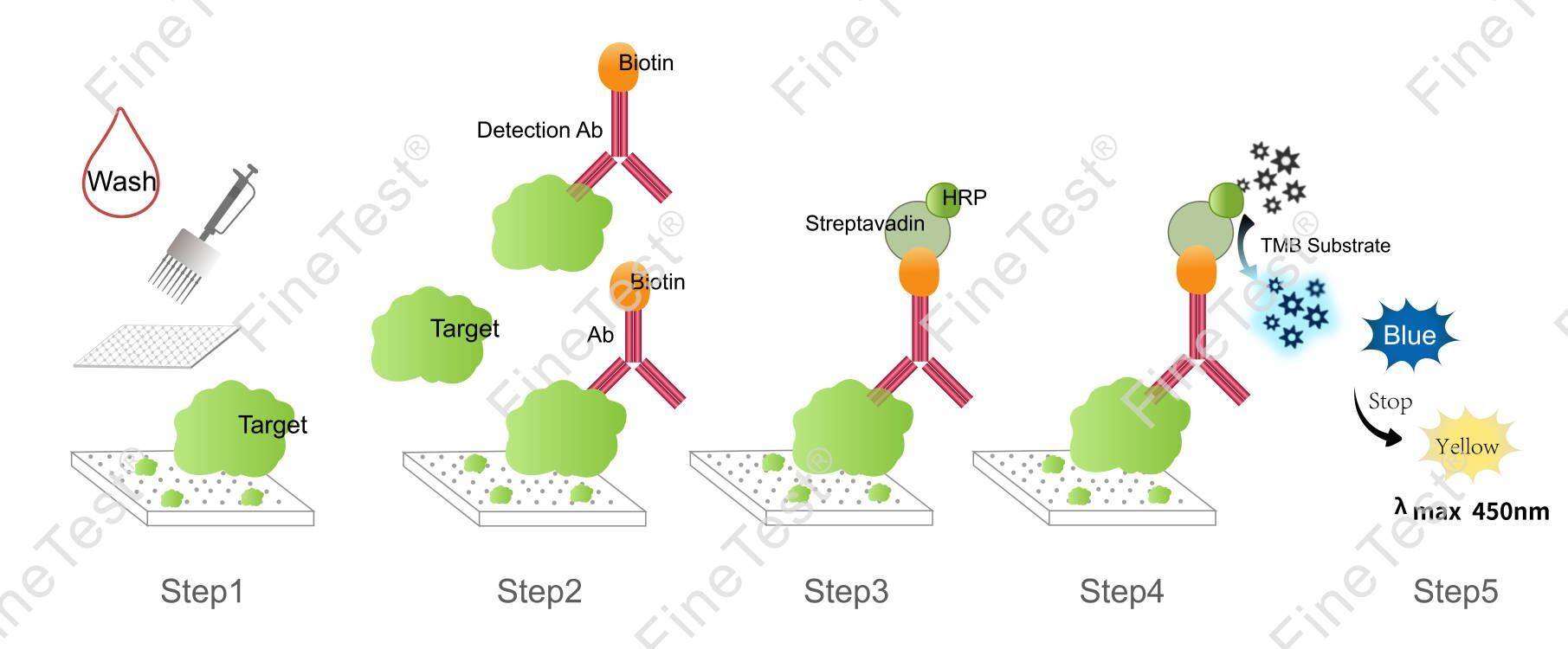
- Step 1: Wash the plate twice before adding the standard and sample.
- Step 2: Add 50ul standard or sample into each well. Immediately add 50ul biotin-labeled antibody working solution into each well(The tip can't be reused after touching the liquid.), gently shake the plate for 1min, seal closely and statically incubate for 45 minutes at 37°C.
- Washing: Wash the plate three times and immerse for 1min each time.
- Step 3: Add 100ul HRP-Streptavidin Conjugate (SABC) working solution into each well, seal the plate and statically incubate for 30 minutes at 37°C.
- Washing: Wash the plate five times and immerse for 1min each time.
- Step 4: Add 90ul TMB substrate solution, seal the plate and statically incubate for 10-20 minutes at 37°C. (Accurate TMB visualization control is required.)
- Step 5: Add 50ul stop solution. Read at 450nm immediately and calculate.
- Standard Curve
-
This product is detected by QC department and meets performance required in the manual. (Laboratory Humidity: 20%-60%; Temperature: 18°C -25°C; Equilibrate TMB substrate to 37°C before staining. After adding into the ELISA wells, incubate for 15min at 37°C in dark.)
Due to different assay environments and operations, assay data below and standard curve are provided for reference. Experimenters should establish standard curve according to their own assay.
STD.(mIU/ml) OD-1 OD-2 Average 0 2.345 2.465 2.393 0.313 1.91 1.988 1.949 0.625 1.559 1.622 1.59 1.25 1.324 1.378 1.351 2.5 0.949 0.987 0.968 5 0.67 0.697 0.684 10 0.336 0.35 0.343 20 0.209 0.217 0.213 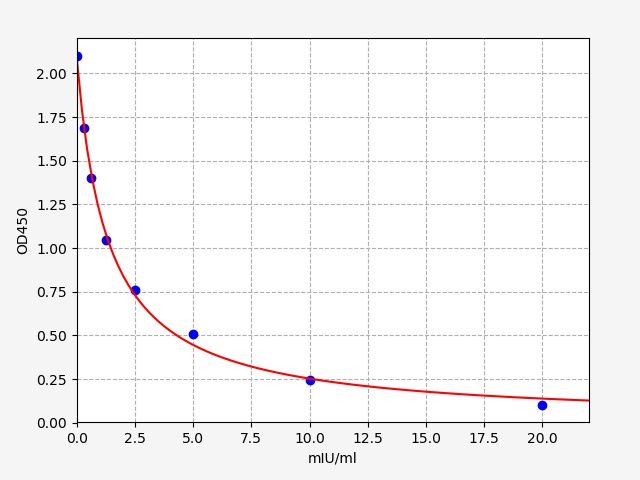
- Recovery
-
Add a certain amount of LH into the sample. Calculate the recovery by comparing the measured value with the expected amount of LH in the sample.
Sample Type Recovery Range(%) Average(%) serum(n=10) 87-101 94 EDTA plasma(n=10) 88-105 100 Heparin plasma(n=10) 97-103 99 - Linearity
-
Dilute the sample with a certain amount of LH at 1:2, 1:4 and 1:8 to get the recovery range.
Sample Type 1:2 1:4 1:8 serum(n=10) 85-104% 86-99% 89-105% EDTA plasma(n=10) 82-97% 82-99% 84-101% Heparin plasma(n=10) 80-99% 87-100% 83-100% - Precision(%)
-
Intra-assay Precision: samples with low, medium and high concentration are tested 20 times on the same plate.
Inter-assay Precision: samples with low, medium and high concentration are tested 20 times on three different plates.
Item Intra-assay Precision Inter-assay Precision Sample 1 2 3 1 2 3 n 20 20 20 20 20 20 Mean (mIU/ml) 0.58 2.35 9.54 0.66 2.51 9.84 Standard deviation 0.03 0.14 0.54 0.03 0.12 0.51 CV(%) 4.89 5.82 5.68 5.12 4.68 5.22 - Stability
-
Perform the stability test for the sealed kit at 37°C and 2-8°C and get relevant data.
ELISA kit(n=5) 37°C for 1 month 2-8°C for 6 months Average(%) 80 95-100
- Journal:
- Science of The Total Environment
- Author:
- Department of Toxicology “Akademik Danilo Soldatovi?”, University of Belgrade, Faculty of Pharmacy, Vojvode Stepe 450, 11221 Belgrade, Serbia
- Cited Date:
- 2023-07-14
- Product:
- Journal:
- Nutrients
- Cited Date:
- 2022-10-20
- Product:
- Journal:
- Antioxidants
- Author:
- Pathophysiology, Department 2—Functional Sciences, Faculty of Medicine, “Iuliu Ha?ieganu” University of Medicine and Pharmacy, 400012 Cluj-Napoca, Romania
- Sample:
- plasma
- Cited Date:
- 2025-05-16
- Product:
-
- Rat T(Testosterone) ELISA Kit
- Rat INS(Insulin) ELISA Kit
- 3-NT(3-Nitrotyrosine) ELISA Kit
- Rat Estrogen ELISA Kit
- Rat 8-OHdG(8-Hydroxydeoxyguanosine) ELISA Kit
- Rat NF-κB(Nuclear Factor Kappa B) ELISA Kit
- Rat LH(Luteinizing Hormone) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat FSH(Follicle Stimulating Hormone) ELISA Kit
- Journal:
- Pakistan Veterinary Journal
- Author:
- Department of Zoology, Lahore College for Women University, Lahore, Pakistan.
- Sample:
- serum
- Cited Date:
- 2025-09-26
- Product:
- Journal:
- Environmental Science and Pollution Research
- Author:
- Department of Zoology, Faculty of Science, Cairo University, Cairo, Egypt
- Cited Date:
- 2023-06-09
- Product:
- Journal:
- International Journal of Molecular Sciences
- Author:
- Department of Pharmacology and Pharmacotherapy, Albert Szent-Gy?rgyi Medical School, University of Szeged, 6725 Szeged, Hungary
- Sample:
- plasma
- Cited Date:
- 2025-06-06
- Product:
- Journal:
- Biology of Sex Differences
- Author:
- Barcelona Liver Bioservices SL, Barcelona, Spain.
- Sample:
- serum
- Cited Date:
- 2025-06-13
- Product:
- Journal:
- International Journal of Molecular Sciences
- Author:
- Pathophysiology, Department 2-Functional Sciences, Faculty of Medicine,"Iuliu Ha?ieganu" University of Medicine and Pharmacy, 400012 Cluj-Napoca, Romania.
- Sample:
- serum
- Cited Date:
- 2025-06-20
- Product:
-
- Rat T(Testosterone) ELISA Kit
- Rat INS(Insulin) ELISA Kit
- 3-NT(3-Nitrotyrosine) ELISA Kit
- Rat Estrogen ELISA Kit
- Rat 8-OHdG(8-Hydroxydeoxyguanosine) ELISA Kit
- Rat NF-κB p65(Nuclear Factor-κB P65) ELISA Kit
- Rat LH(Luteinizing Hormone) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat FSH(Follicle Stimulating Hormone) ELISA Kit
- Journal:
- International Journal of Molecular Sciences
- Author:
- Department of Food Science, National Taiwan Ocean University, Keelung 20224, Taiwan
- Sample:
- plasma
- Cited Date:
- 2025-06-20
- Product:
- Journal:
- European Journal of Pharmacology
- Author:
- Department of Animal Sciences, Faculty of Biological Sciences, Kharazmi University, Tehran, Iran.
- Cited Date:
- 2025-09-05
- Product:
- Journal:
- Frontiers in Endocrinology
- Author:
- Department of Pediatrics, The First Affiliated Hospital of Guangxi Medical University, Nanning,?China.
- Cited Date:
- 2025-12-12
- Product:
- Journal:
- Biomedicine & Pharmacotherapy
- Cited Date:
- 2021-06-04
- Product:
- Journal:
- Molecular and Cellular Endocrinology
- Cited Date:
- 2023-04-14
- Product:
- Journal:
- Food and Chemical Toxicology
- Cited Date:
- 2019-06-03
- Product:
-
- FA/VB9(Folic Acid/Vitamin B9) ELISA Kit
- Rat VB 12(vitamin B12) ELISA Kit
- Rat E2(Estradiol) ELISA Kit
- Rat HCY(Homocysteine) ELISA Kit
- Rat NF-κB(Nuclear Factor Kappa B) ELISA Kit
- Rat LH(Luteinizing Hormone) ELISA Kit
- Rat FSH(Follicle Stimulating Hormone) ELISA Kit
- Rat MT-1(Metallothionein-1) ELISA Kit
- Journal:
- Molecular and Cellular Endocrinology
- Author:
- Department of Obstetrics and Gynecology, The First Affiliated Hospital of the Fourth Military Medical University, Xi'an, China.
- Cited Date:
- 2024-10-08
- Product:
- Journal:
- ACS Omega
- Author:
- Nutrition and Food Sciences Department, National Research Centre, Dokki, Giza 12622, Egypt
- Sample:
- blood sample
- Cited Date:
- 2024-07-12
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat LH(Luteinizing Hormone) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat FSH(Follicle Stimulating Hormone) ELISA Kit
- Rat Gc(Glucagon) ELISA Kit
- Rat Iapp(Islet amyloid polypeptide) ELISA Kit
- Rat Pth(PaRathyroid hormone) ELISA Kit
- Rat Lep(Leptin) ELISA Kit
- Rat IL-10(Interleukin-10) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Journal:
- Biochemical and Biophysical Research Communications
- Cited Date:
- 2022-10-14
- Product:
- Journal:
- Reproductive Toxicology
- Author:
- Department of Gynecology, First Affiliated Hospital, Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang, China.
- Cited Date:
- 2025-04-18
- Product:
- Journal:
- Environmental Science and Pollution Research
- Cited Date:
- 2021-05-07
- Product:
- Journal:
- Reproductive Sciences
- Author:
- Clinical Nutrition and Dietetics, Department of Biomedical Laboratory Science and Management (UGC Innovative Department), Vidyasagar University, Midnapore, West Bengal, 721 102, India
- Cited Date:
- 2023-07-14
- Product:
- Journal:
- Endocrine Connections
- Author:
- Department of Pediatrics, The First Affiliated Hospital of Guangxi Medical University, Nanning, China.
- Cited Date:
- 2025-11-28
- Product:
- Journal:
- Andrologia
- Cited Date:
- 2022-04-28
- Product:
- Journal:
- PLOS ONE
- Author:
- Department of Pharmacology and Pharmacotherapy, Albert Szent-Gy?rgyi Medical School, University of Szeged, Szeged, Hungary.
- Sample:
- plasma
- Cited Date:
- 2024-08-16
- Product:
- Journal:
- Tissue and Cell
- Author:
- Department of Medical Biochemistry, Faculty of Medicine, Karadeniz Technical University, 61080 Trabzon, Turkey.
- Cited Date:
- 2024-07-12
- Product:
- Journal:
- Journal of the Science of Food and Agriculture
- Cited Date:
- 2020-09-01
- Product:
- Journal:
- Drug and Chemical Toxicology
- Author:
- Department of Nutrition and Dietetics, Faculty of Health Sciences, Karadeniz Technical University, Trabzon, Turkey
- Cited Date:
- 2023-06-02
- Product:
- Journal:
- Biochemical and Biophysical Research Communications
- Author:
- Department of Histology and Embryology, Faculty of Medicine, Istinye University, Istanbul, Turkey
- Cited Date:
- 2025-02-28
- Product:
- Journal:
- Clinical and Experimental Pharmacology and Physiology
- Author:
- Department of Gynecology, First Affiliated Hospital, Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang, China.
- Cited Date:
- 2025-07-25
- Product:
- Journal:
- Neuroscience Letters
- Author:
- The Fourth Department of Surgery, First Affiliated Hospital, Heilongjiang University of Chinese Medicine, Harbin, Heilongjiang, People's Republic of China.
- Cited Date:
- 2025-05-23
- Product:
-
- DA(Dopamine) ELISA Kit
- NA/NE(Noradrenaline/Norepinephrine) ELISA Kit
- Rat fT3(Free Triiodothyronine) ELISA Kit
- Rat 5-hydroxytryptamine ELISA Kit
- Rat TSH(Thyroid Stimulating Hormone) ELISA Kit
- Rat LH(Luteinizing Hormone) ELISA Kit
- Rat FSH(Follicle Stimulating Hormone) ELISA Kit
- Rat fT4(Free Thyroxine) ELISA Kit
- Journal:
- Biological Trace Element Research
- Cited Date:
- 2017-06-08
- Product:
- Journal:
- International Urology and Nephrology
- Author:
- Department of Nutrition and Dietetics, Faculty of Health Sciences, Karadeniz Technical University, 61080, Trabzon, Turkey
- Cited Date:
- 2023-10-08
- Product:
- Journal:
- Cell Biochemistry and Biophysics
- Author:
- Department of Histology and Embryology, Faculty of Medicine, Hatay Mustafa Kemal University, Hatay, Turkey.
- Cited Date:
- 2024-09-20
- Product:
- Journal:
- JBRA Assisted Reproduction
- Author:
- Behbahan Faculty of Medical Sciences, Behbahan, Iran.
- Sample:
- serum
- Cited Date:
- 2024-03-22
- Product:
- Journal:
- Journal of Advanced Pharmacy Education & Research
- Cited Date:
- 2019-11-22
- Product:
- Journal:
- Food Science and Biotechnology
- Cited Date:
- 2018-01-08
- Product:
- Journal:
- Egyptian Pharmaceutical Journal
- Author:
- Department of Biotechnology, Faculty of Science, Cairo University, Giza, Egypt
- Sample:
- serum
- Cited Date:
- 2024-12-27
- Product:
- Journal:
- Journal of Basic and Clinical Physiology and Pharmacology
- Cited Date:
- 2019-06-13
- Product:
-
- FA/VB9(Folic Acid/Vitamin B9) ELISA Kit
- Rat VB 12(vitamin B12) ELISA Kit
- Rat E2(Estradiol) ELISA Kit
- Rat HCY(Homocysteine) ELISA Kit
- Rat NF-κB(Nuclear Factor Kappa B) ELISA Kit
- Rat LH(Luteinizing Hormone) ELISA Kit
- Rat FSH(Follicle Stimulating Hormone) ELISA Kit
- Rat MT-1(Metallothionein-1) ELISA Kit
- Journal:
- International Journal of Reproductive BioMedicine
- Cited Date:
- 2022-03-25
- Product:
- Journal:
- International Journal of Complementary & Alternative Medicine
- Cited Date:
- 2022-09-02
- Product:
- Journal:
- J Ardabil Univ Med Sci
- Cited Date:
- 2016-12-30
- Product:
- Journal:
- Fisheries and Aquatic Sciences
- Cited Date:
- 2021-11-25
- Product:
- Journal:
- Egyptian Academic Journal of Biological Sciences
- Author:
- Department of Anatomy and Embryology, Faculty of Medicine, Ain-Shams University, Abbassia, Postal code: 11591 .Cairo, Egypt.
- Cited Date:
- 2024-02-18
- Product:
- Journal:
- Proceedings of the Zoological Society
- Author:
- Department of Allied Health Science, Brainware University, Barasat, Kolkata, West Bengal, 700125, India
- Cited Date:
- 2024-02-18
- Product:
- Journal:
- Journal of Menopausal Medicine
- Author:
- Department of Medical Biology, Faculty of Medicine, Firat University, Elaz??, Türkiye.
- Sample:
- serum
- Cited Date:
- 2024-09-27
- Product:
- Journal:
- Bayero Journal of Pure and Applied Sciences
- Cited Date:
- 2021-12-23
- Product:
- Journal:
- FACETS
- Cited Date:
- 2018-07-12
- Product:
- Journal:
- FUW Trends in Science & Technology Journal
- Author:
- Department of Human Physiology, Faculty of Basic Medical Science, Ahmadu Bello University, Zaria, Nigeria.
- Sample:
- serum
- Cited Date:
- 2024-07-26
- Product:
- Journal:
- FLUORIDE
- Cited Date:
- 2019-12-19
- Product:
- Journal:
- Egyptian Journal of Chemistry
- Author:
- Biotechnology/Biomolecular Chemistry Department, Faculty of Science, Cairo University, Egypt
- Cited Date:
- 2024-03-01
- Product:
- Journal:
- Tropical Journal of Obstetrics and Gynaecology
- Author:
- Federal Teaching Hospital Katsina
- Cited Date:
- 2025-10-17
- Product:
- Journal:
- 15th International Conference on Computing Communication and Networking Technologies
- Author:
- Dept. of Allied Health Sciences Brainware University Kolkata, India
- Cited Date:
- 2025-01-10
- Product:
- Journal:
- Benha Veterinary Medical Journal
- Cited Date:
- 2022-12-15
- Product:
- Journal:
- Benha Veterinary Medical Journal
- Cited Date:
- 2022-12-29
- Product:
What’s the plate size in FineTest® ELISA Kits?
The ELISA plate follows the standard size of microplate: 127.64 mm x 85.60 mm x 14.22 mm(L x W x H).
How about the shelf life and stability of FineTest® ELISA Kits?
Valid for 12 months since the production date. For the shelf life of specific batch number, please check the label printed on the kit. Before delivery, all FineTest® ELISA Kits have been subject to strict quality test.
Which cloned antibodies for FineTest® ELISA Kits are used?
These information is proprietary. Please contact us to learn more about clonality (polyclonality or monoclonality) and host species.
Can I mix reagents from different batches of FineTest® ELISA Kits?
Not suggested. ELISA reagents are optimized for specific batch.
Can FineTest® ELISA Kits be used partially?
Yes. The ELISA plate is dismounted. Enough component volumes are offered by 96T ELISA kit, supporting two groups of standard curve.
How long can the diluted lyophilized standard be stored for continual use?
Used up within 12h.
Can standard curve be extended to any direction?
FineTest® can't support validation of standard concentration outside of standard curve. Ranges of standard curve have been validated among many batches and experimenters, showing stable and accurate performance. The lowest standard concentration is the minimized range for reliable detection results. Adding higher or lower concentration of standard may cause inconsistent signal or false positive.
Why does detection for serum/plasma sample by FineTest® ELISA Kits require for 1/2 dilution?
Matrix components in serum/plasma can affect detection results. Blocking components in sample dilution buffer can decrease or remove the interference. The dilution can reduce the matrix difference between sample and standard to get better accuracy.
What’s the half-life of protein in serum/plasma/cell culture supernatant?
FineTest® can't determine the half-life of protein in the sample(e.g. serum, plasma or cell culture supernatant). Usually, it's suggested to detect prepared sample immediately or aliquot sample to refrigerate in a disposable container. Avoid freeze-thaw cycle to prevent protein degradation.
What's the expected concentration for particularly analyzing my sample?
Due to the specificity of each sample, it's hard to forecast and depend on sample preparation as well as analytical characteristics. Please contact us to get detection data for reference.
How many samples can FineTest® ELISA Kits detect?
It depends on whether duplicate assay for your sample is required. Such as, you can detect 80 samples without duplication or 40 samples.
Why is sample loading conducted after equilibrating all kits’ components and samples to room temperature?
Temperature is the important factor for ELISA binding reaction. The reaction of samples at a consistent temperature requires for equilibrating all reagents to room temperature before the assay, including tested samples. Avoid the inaccurate ELISA assay results caused by temperature differences.
Why is duplicate assay suggested for ELISA assay?
To get more accurate assay results, it's strongly suggested to conduct duplicate assay for the standard and sample. Duplicate assay can calculate average value to ensure more accurate assay results and solve drift caused by misoperation during the assay. Calculation of CV evaluates experimental operation and precision of the ELISA kit.
How to get the standard curve with excellent linearity?
Follow the suggested method in the manual to store the standard. Before dissolving the standard, transient centrifugation is required to completely collect the powder. Confirm the fully dissolution and mixing of the standard(about 10min). Then, conduct steps of series dilution. Ensure the fully mixing and accurate pipetting in each step. Properly stop the staining. Choose suitable fitting equation to plot the standard curve.
Can ELISA plates be stacked together for incubation?
To keep the consistent environmental condition of all plates, stacking is not suggested during incubation.
Why is polypropylene tube used for standard dilution in some analyses?
Some proteins or analytes can bind with glass or polystyrene. However, they are not easy to bind with polypropylene tube.
When to stop ELISA reaction?
ELISA assay finally requires for enzyme-catalyzed substrate to complete the staining reaction. Stopping the reaction at the best time is the important factor for successful ELISA assay. When the blue complex appears after adding TMB substrate for 12min, read the O.D. absorbance at 620nm. The staining can be stopped, when the OD value of the darkest color at 620nm is between 0.8 - 0.9. The relevant OD value at 450nm for stopping the staining is between 2.0 - 2.5. If the OD value at 620nm is lower than 0.8, the staining duration can be properly extended.
Why is dual wavelength selected for reading in a microplate reader? What's the purpose of wavelength correction?
Dual wavelength for reading in ELISA assay mainly aims to remove non-specific background interference, and improve accuracy and reliability of the detection. FineTest® usually suggests to set the corrected wavelength as 570nm or 630nm in a microplate reader. The OD value may be higher when directly reading at 450nm. However, the accuracy is lower.
Why is 4 - pl curve fitting is required for generating standard curve?
FineTest® recommends to use ELISA curve fitting software and validate ELISA kit with 4 - pl curve fitting. 4 - pl curve fitting mainly aims to accurately describe the nonlinear dynamic characteristics of antigen - antibody binding. Thus, the quantitative accuracy of low or high concentration of sample is improved. Experimental errors are decreased. The reliability and reproducibility of assay results are ensured.
If sample OD value is higher than OD value of the highest point on the standard curve, what's the suggested solution?
Dilute the sample with dilution buffer and conduct the detection again. Ensure the detected value of the sample falls in the range of standard curve.
Are there any rewards to publish papers using FineTest® ELISA Kits?
If you published paper using FineTest® ELISA Kits, you will obtain US$150-US$550 coupon, please contact us for more details.