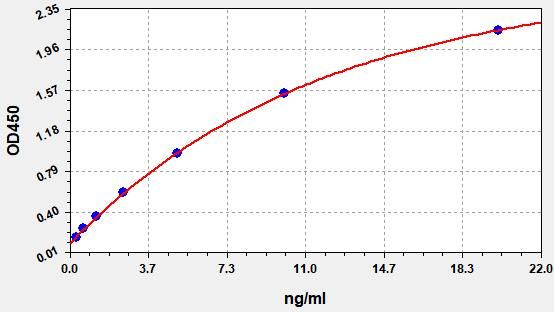
| Catalogue No.: | ER2094 | Species: | Rat |
| Range: | 1.25-80ng/ml | Sensitivity: | 0.75ng/ml |
Polyethylene glycol (PEG) is a polymer material that is widely used in the modification of biopolymers such as proteins and peptides because of its non-toxicity, good biocompatibility and amphiphilicity. Proteins, enzymes or peptides modified by PEGylation can be effectively stabilized , the half-life of the drug can be significantly extended and the immunogenicity of the protein drug can be weakened. Currently, more and more PEG-modified protein drugs are approved for clinical treatment or are in preclinical development.
In recent years, more and more studies have shown that PEG and PEG-modified protein drugs can produce anti-PEG antibodies (APA) in animals or humans, and the strength of APA titre is related to drug clearance. The increase of APA titre to a certain level causes accelerated blood clearance (ABC), thus affecting the efficacy of the drug treatment and resulting in adverse drug reactions (ADR). Patients generally have higher titres of IgG antibodies and IgM antibodies to APA, and patients with high titres of IgM antibodies appear earlier.
APA is also produced in normal individuals and in more than 25% of patients who have not been treated with PEG-modified drugs. If these patients are treated with PEG-modified protein drugs, the efficacy of the drugs may be reduced and the desired result may not be achieved. Therefore the detection and analysis of APA is very important.
FineTest® has developed the following products to aid in APA testing.
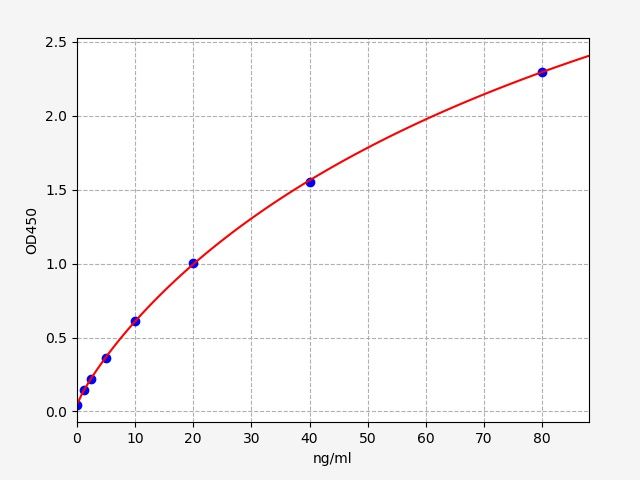
| Catalogue No.: | ER2094 | Species: | Rat |
| Range: | 1.25-80ng/ml | Sensitivity: | 0.75ng/ml |

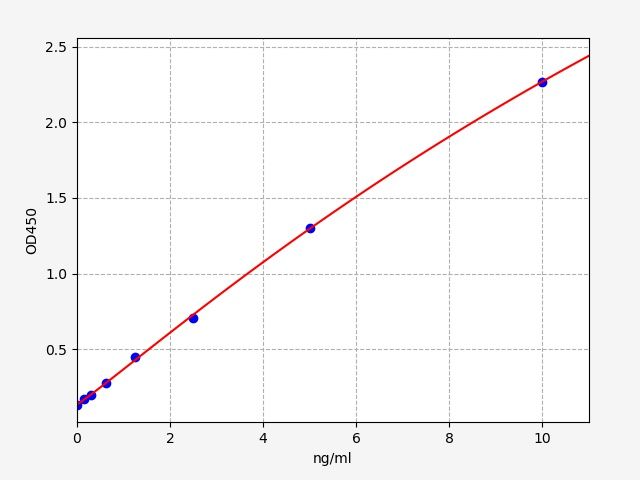
| Catalogue No.: | ER2095 | Species: | Rat |
| Range: | 0.156-10ng/ml | Sensitivity: | 0.094ng/ml |
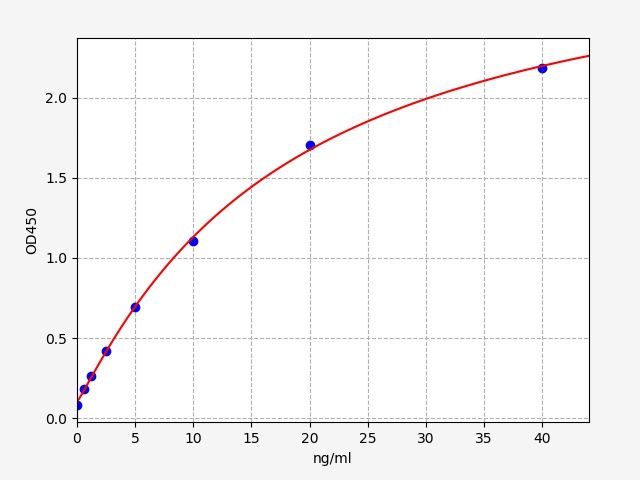
| Catalogue No.: | ERB0231 | Species: | Rabbit |
| Range: | 0.625-40ng/ml | Sensitivity: | 0.375ng/ml |
| Catalogue No.: | ERB0232 | Species: | Rabbit |
| Range: | 0.156-10ng/ml | Sensitivity: | 0.094ng/ml |
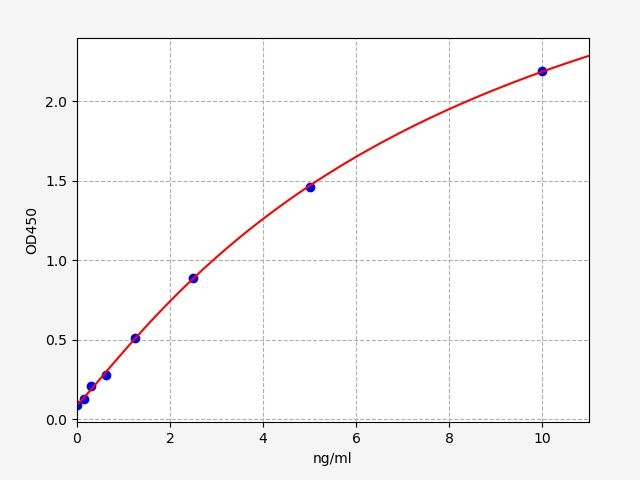
| Catalogue No.: | EM2149 | Species: | Mouse |
| Range: | 0.781-50ng/ml | Sensitivity: | 0.469ng/ml |
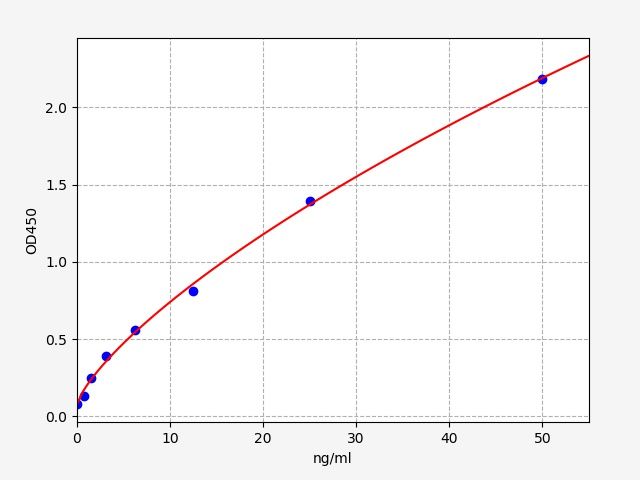
| Catalogue No.: | EM2150 | Species: | Mouse |
| Range: | 0.313-20ng/ml | Sensitivity: | 0.188ng/ml |
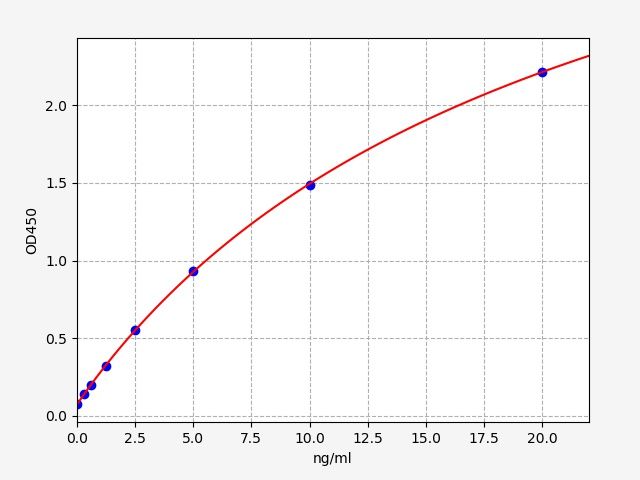
| Catalogue No.: | EMK0221 | Species: | Monkey |
| Range: | 0.625-40ng/ml | Sensitivity: | 0.375ng/ml |
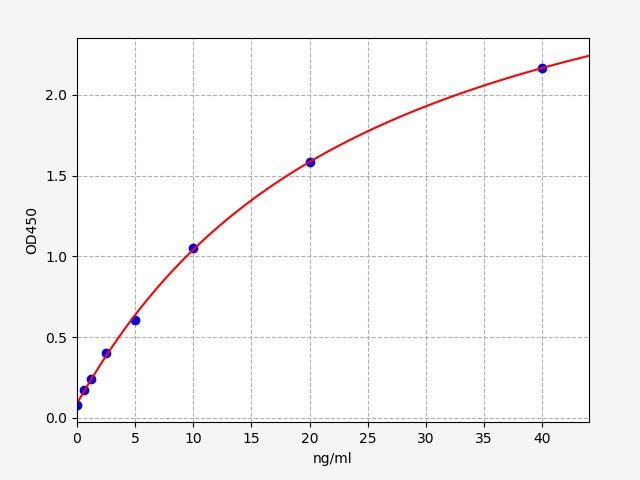
| Catalogue No.: | EMK0222 | Species: | Monkey |
| Range: | 0.313-20ng/ml | Sensitivity: | 0.188ng/ml |
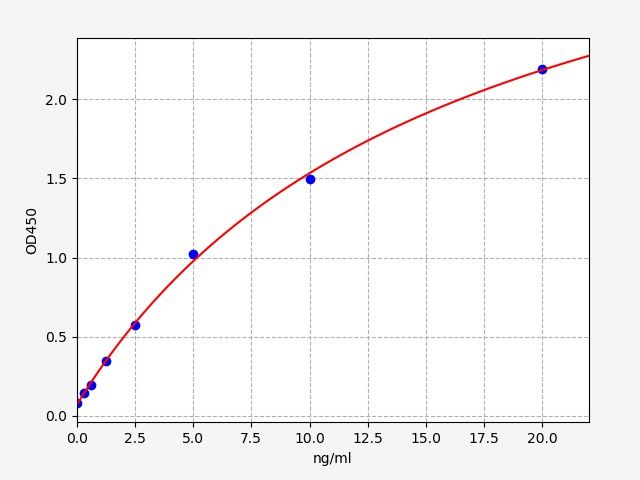
| Catalogue No.: | EH5111 | Species: | Human |
| Range: | 0.625-40ng/ml | Sensitivity: | 0.375ng/ml |
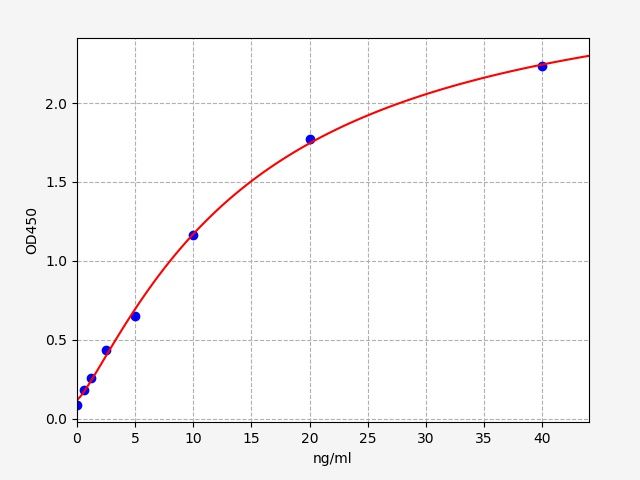
| Catalogue No.: | EH5112 | Species: | Human |
| Range: | 0.313-20ng/ml | Sensitivity: | 0.188ng/ml |