Products
Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
This kit is based on Double antibody-Sandwich ELISA detection method and takes 4h assay time. The microplate provided in this kit has been precoated with anti TNF-α antibody. Add standard and properly diluted sample into relevant well respectively. After incubation, wash unbound components. Add biotinylated detection antibody. Then, it binds with TNF-α bound to precoated antibody. Wash unbound components and add HRP-Streptavidin Conjugate (SABC). Wash unbound components again and add TMB substrate solution. Then, TMB was catalyzed by HRP to produce a blue color product that turned yellow after adding a stop solution. Read the O.D. absorbance at 450nm in a microplate reader. Calculate the concentration of TNF-α in the sample by plotting standard curve. The concentration of the target substance is proportional to the OD450 value.
- Catalogue No.:
- EH0302
- Alias:
- Tumor necrosis factor ELISA Kit, Cachectin ELISA Kit, TNF-alpha ELISA Kit, Tumor necrosis factor ligand superfamily member 2 ELISA Kit, TNF-a ELISA Kit, Tumor necrosis factor, membrane form ELISA Kit, N-terminal fragment ELISA Kit, NTF ELISA Kit, Intracellular domain 1 ELISA Kit, ICD1 ELISA Kit, Intracellular domain 2 ELISA Kit, ICD2 ELISA Kit, C-domain 1 ELISA Kit, C-domain 2 ELISA Kit, Tumor necrosis factor, soluble form ELISA Kit, TNF ELISA Kit, TNFA ELISA Kit, TNFSF2 ELISA Kit
- Species:
- Human
- Range:
- 15.625-1000pg/ml (1pg=76mIU)
- Sensitivity:
- 9.375pg/ml
| Size | Price |
|---|---|
| 96T | Inquiry |
| 48T | Inquiry |
- SPECIFICATIONS
- CITATIONS
- FIGURES
- CONDITIONS
- FAQS
- Product Name
- Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Alias
- Tumor necrosis factor ELISA Kit, Cachectin ELISA Kit, TNF-alpha ELISA Kit, Tumor necrosis factor ligand superfamily member 2 ELISA Kit, TNF-a ELISA Kit, Tumor necrosis factor, membrane form ELISA Kit, N-terminal fragment ELISA Kit, NTF ELISA Kit, Intracellular domain 1 ELISA Kit, ICD1 ELISA Kit, Intracellular domain 2 ELISA Kit, ICD2 ELISA Kit, C-domain 1 ELISA Kit, C-domain 2 ELISA Kit, Tumor necrosis factor, soluble form ELISA Kit, TNF ELISA Kit, TNFA ELISA Kit, TNFSF2 ELISA Kit
- Catalogue No.
- EH0302
- Size
- 48T/96T
- Species
- Human
- UniProt ID
- P01375
- Sample Type
- Serum, Plasma, Cell Culture Supernatant, cell or tissue lysate, Other liquid samples
- Detection Method
- Sandwich ELISA, Double Antibody
- Detection Wavelength
- OD450
- Reaction Duration
- 4 hours
- Range
- 15.625-1000pg/ml (1pg=76mIU)
- Sensitivity
- 9.375pg/ml
- Storage
- 2-8°C(Sealed), Don't cryopreserve.
- Specificity
- Specifically binds with TNF-α , no obvious cross reaction with other analogues.
- Recommended Sample Dilution Ratio
-
The following table shows the recommended dilution ratios for this kit for a limited number of samples for your reference only. (The matrix components in serum/plasma will affect the test results, which it need to be diluted at least 1/2 with Sample Dilution Buffer before testing! When the content of other samples is very low, the original solution can be added without dilution, but it is necessary to ensure that the pH is between 6.8 and 8.0, and it does not contain more than 10% organic solvents or high-concentration protein denaturants.)
1. Common sample validation:
Sample Type Recommended Dilution Ratio Content Healthy human serum/plasma (EDTA, Citrate , heparin) (n=10) 1/2 dilution ND-19pg/ml Serum of patients with bacterial infection (CRP >7ug/ml) (n=4) 1/2-1/5 dilution 96-695pg/ml Plasma of patients with bacterial infection (CRP >7ug/ml) (n=4) 1/2-1/5 dilution 124-795pg/ml THP-1 cells were treated with 80nM TPA and cultured overnight. Take the cell culture supernatant for detection. 1/2 dilution ND THP-1 cells were treated with 80nM TPA and cultured overnight. Then, stimulate with 100ng/ml LPS for 6 hours and take the cell culture supernatant for detection. 1/20 dilution 2.3ng/ml THP-1 cells were treated with 80nM TPA and cultured overnight. Then, they were stimulated with 100ng/ml LPS for 6 hours. After that, 300ng/ml Brefeldin A (BFA) was added and cultured for 3 hours. The cell lysate (Cat No: E050) was collected, and the total protein concentration of 1.2mg/ml was detected by BCA. 1/10 dilution 652pg/mg(total protein) 2. KO sample validation (Detect TNF-α KO THP-1 cells):
Sample Type Dilution Ratio Content Wild THP-1 cells were treated with 80nM TPA and cultured overnight, and then stimulated with 200ng/ml LPS for 6 hours. The cell culture supernatant was taken for detection. 1/20 dilution 1.5ng/ml KO THP-1 cells were treated with 80nM TPA and cultured overnight, and then stimulated with 200ng/ml LPS for 6 hours. The cell culture supernatant was taken for detection. 1/2 dilution ND KO THP-1 cells were treated with 80nM TPA and cultured overnight. Then they were stimulated with 200ng/ml LPS for 6 hours. After that, 300ng/ml Brefeldin A (BFA) was added and cultured for 3 hours. The cell lysate was collected (Cat No: E050), and the total protein concentration of 0.92mg/ml was detected by BCA. 1/2 dilution ND Note:ND is lower than the sensitivity of the kit and was not detected
3. Capture antibody and detection antibody by WB KO validation (Detect TNF-α KO THP-1 cells):
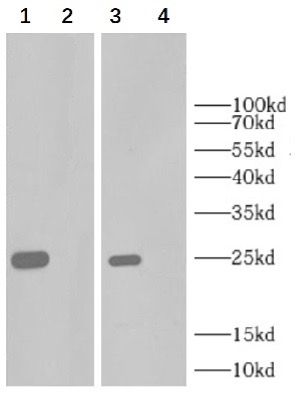 1 and 3: Wild THP-1 cells were treated with 80nM TPA, cultured overnight, stimulated with 200ng/ml LPS for 6 hours, then cultured with 300ng/ml Brefeldin A (BFA) for 3 hours, and the cell lysate was collected (Cat No: E050), and the total protein concentration of 1.55mg/ml was detected by BCA, with 10ug of total protein loading.
1 and 3: Wild THP-1 cells were treated with 80nM TPA, cultured overnight, stimulated with 200ng/ml LPS for 6 hours, then cultured with 300ng/ml Brefeldin A (BFA) for 3 hours, and the cell lysate was collected (Cat No: E050), and the total protein concentration of 1.55mg/ml was detected by BCA, with 10ug of total protein loading.
2 and 4: KO THP-1 cells were treated with 80nM TPA, cultured overnight, stimulated with 200ng/ml LPS for 6 hours. then, then cultured with 300ng/ml Brefeldin A (BFA) for 3 hours, and the cell lysate was collected (Cat No: E050), and the total protein concentration of 1.55mg/ml was detected by BCA, with 10ug of total protein loading.
Primary antibody:
12:Capture mouse monoclonal antibody 1ug/ml
34:Detection mouse monoclonal antibody 1ug/ml
secondary antibody:
1234 : HRP-Goat Anti-mouse IgG (H&L) (Catalogue No.:FNSA-0003) at 1/5000 dilution
Molecular weight: 26 kDa4. Verified recombinant protein:
(It is a normal phenomenon that some proteins have weak detection signals or cannot be detected at all due to differences in tags, sequences or protein activities)
A. HEK293-derived human TNF-alpha protein(Val77-Leu233) activity
B. E.coli-derived human TNF-alpha protein(Val77-Leu233) with and without an N-terminal Met - ELISA Kit Components
Kit Components Item Size(48T) Size(96T) Storage Condition for Opened Kit E001 ELISA Microplate(Dismountable) 8×6 8×12 Put the rest strips into a sealed foil bag with the desiccant. Stored for 1 month at 2-8°C; Stored for 6 month at -20°C E002 Lyophilized Standard 1vial 2vial Put the rest standards into a desiccant bag. Stored for 1 month at 2-8°C; Stored for 6 month at -20°C E003 Biotin-labeled Antibody(Concentrated, 100X) 60ul 120ul 2-8°C (Avoid Direct Light) E034 HRP-Streptavidin Conjugate(SABC, 100X) 60ul 120ul E024 TMB Substrate 5ml 10ml E039 Sample Dilution Buffer 10ml 20ml 2-8°C E040 Antibody Dilution Buffer 5ml 10ml E049 SABC Dilution Buffer 5ml 10ml E026 Stop Solution 5ml 10ml E038 Wash Buffer(Concentrated, 25X) 15ml 30ml E006 Plate Sealer 3 pieces 5 pieces E007 Product Description 1 copy 1 copy - Required Instruments and Reagents
-
- Microplate reader (wavelength: 450nm)
- 37°C incubator (CO2 incubator for cell culture is not recommenced.)
- Automated plate washer or multi-channel pipette/5ml pipettor (for manual washing purpose)
- Precision single (0.5-10μL, 5-50μL, 20-200μL, 200-1000μL) and multi-channel pipette with disposable tips(Calibration is required before use.)
- Sterile tubes and Eppendorf tubes with disposable tips
- Absorbent paper and loading slot
- Deionized or distilled water
- Assay Procedure Summary
-
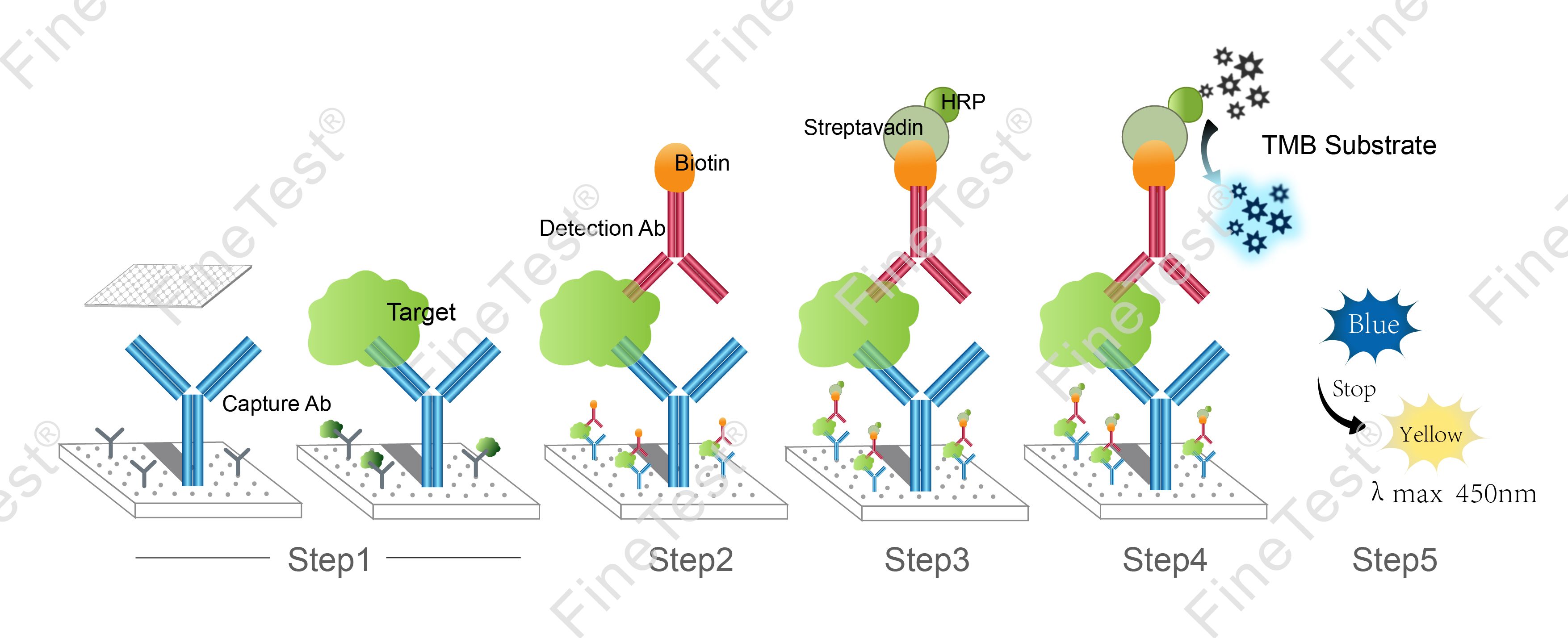
- Step 1: Add 100ul standard or sample into each well, seal the plate and statically incubate for 90 minutes at 37°C.
- Washing: Wash the plate twice without immersing.
- Step 2: Add 100ul biotin-antibody working solution, seal the plate and statically incubate for 60 minutes at 37°C.
- Washing: Wash the plate three times and immerse for 1min each time.
- Step 3: Add 100ul HRP-Streptavidin Conjugate (SABC) working solution, seal the plate and statically incubate for 30 minutes at 37°C.
- Washing: Wash the plate five times and immerse for 1min each time.
- Step 4: Add 90ul TMB substrate solution, seal the plate and statically incubate for 10-20 minutes at 37°C. (Accurate TMB visualization control is required.)
- Step 5: Add 50ul stop solution. Read at 450nm immediately and calculate.
- Standard Curve
-
This product is detected by QC department and meets performance required in the manual. (Laboratory Humidity: 20%-60%; Temperature: 18°C -25°C; Equilibrate TMB substrate to 37°C before staining. After adding into the ELISA wells, incubate for 15min at 37°C in dark.)
Due to different assay environments and operations, assay data below and standard curve are provided for reference. Experimenters should establish standard curve according to their own assay.
STD.(pg/ml) OD-1 OD-2 Average 0 0.142 0.149 0.145 15.625 0.187 0.197 0.191 31.25 0.246 0.258 0.251 62.5 0.302 0.317 0.308 125 0.422 0.444 0.431 250 0.746 0.784 0.761 500 1.256 1.32 1.281 1000 2.05 2.154 2.092 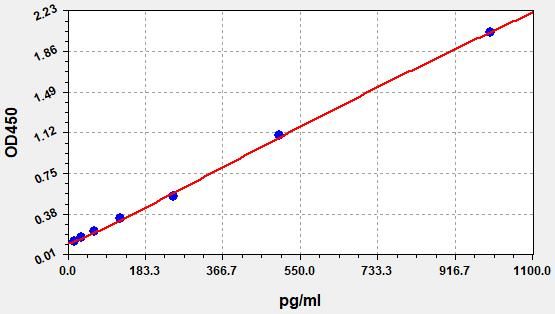
- Recovery
-
Add a certain amount of TNF-α into the sample. Calculate the recovery by comparing the measured value with the expected amount of TNF-α in the sample.
Sample Type Recovery Range(%) Average(%) serum(n=10) 92-103 95 EDTA plasma(n=10) 92-99 93 Heparin plasma(n=10) 86-99 94 - Linearity
-
Dilute the sample with a certain amount of TNF-α at 1:2, 1:4 and 1:8 to get the recovery range.
Sample Type 1:2 1:4 1:8 serum(n=10) 81-93% 82-95% 86-95% EDTA plasma(n=10) 86-103% 84-100% 83-94% Heparin plasma(n=10) 85-96% 80-95% 81-99% - Precision(%)
-
Intra-assay Precision: samples with low, medium and high concentration are tested 20 times on the same plate.
Inter-assay Precision: samples with low, medium and high concentration are tested 20 times on three different plates.
Item Intra-assay Precision Inter-assay Precision Sample 1 2 3 1 2 3 n 20 20 20 20 20 20 Mean (pg/ml) 31.68 118.4 485 32.21 125.2 494 Standard deviation 1.76 7.09 28.71 1.78 7.32 27.12 CV(%) 5.56 5.99 5.92 5.53 5.85 5.49 - Stability
-
Perform the stability test for the sealed kit at 37°C and 2-8°C and get relevant data.
ELISA kit(n=5) 37°C for 1 month 2-8°C for 6 months Average(%) 80 95-100
- Journal:
- Annals of the Rheumatic Diseases
- Author:
- Department of Rheumatology and Immunology, West China Hospital, Sichuan University, Chengdu, Sichuan, China
- Sample:
- serum
- Cited Date:
- 2024-03-08
- Product:
- Journal:
- Journal of Hepatology
- Author:
- Department of Hepatology, Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh, India.
- Cited Date:
- 2025-03-14
- Product:
- Journal:
- Chemical Engineering Journal
- Author:
- State Key Laboratory of Oral & Maxillofacial Reconstruction and Regeneration, National Clinical Research Center for Oral Diseases, Shaanxi International Joint Research Center for Oral Diseases, Department of Periodontology, School of Stomatology, The Four
- Sample:
- supernatants
- Cited Date:
- 2025-03-07
- Product:
- Journal:
- Cancer Research
- Author:
- Fudan University Shanghai Cancer Center, Shanghai, China.
- Sample:
- supernatant
- Cited Date:
- 2024-09-20
- Product:
- Journal:
- Advanced Healthcare Materials
- Author:
- Center for Nanoparticle Research, Institute for Basic Science (IBS), Seoul, 08826, Republic of Korea.
- Cited Date:
- 2025-04-11
- Product:
-
- Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Human IL-6(Interleukin 6) ELISA Kit
- Human COLI(Collagen Type I) ELISA Kit
- Human VEGF(Vascular Endothelial Cell Growth Factor) ELISA Kit
- Human TGF-β1 (Transforming Growth Factor Beta 1) ELISA Kit
- Human PDGF-BB(Platelet Derived Growth Factor-BB) ELISA Kit
- Human IL-1β(Interleukin 1 Beta) ELISA Kit
- Human EGF(Epidermal Growth Factor) ELISA Kit
- Journal:
- Applied Materials Today
- Author:
- Department of Bioengineering, Faculty of Engineering, Ege University, 35100 Izmir, Turkey
- Cited Date:
- 2023-12-22
- Product:
- Journal:
- Materials and Design
- Author:
- NMPA Key Laboratory for Quality Research and Control of Tissue Regenerative Biomaterial, & Institute of Regulatory Science for Medical Device, & National Engineering Research Center for Biomaterials, Sichuan University, Chengdu 610064, China
- Sample:
- supernatants
- Cited Date:
- 2024-07-19
- Product:
-
- Porcine GLA (Alpha-galactosidase A)ELISA Kit
- GAGs(Glycosaminoglycan)ELISA Kit
- Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Human IL-6(Interleukin 6) ELISA Kit
- Human COLI(Collagen Type I) ELISA Kit
- Human VEGF(Vascular Endothelial Cell Growth Factor) ELISA Kit
- Human MMP-9(Matrix Metalloproteinase 9) ELISA Kit
- Journal:
- Frontiers in Immunology
- Author:
- Museum and Institute of Zoology, Polish Academy of Science, Warsaw, Poland.
- Sample:
- hemolymph samples
- Cited Date:
- 2024-05-24
- Product:
-
- Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Human IL-6(Interleukin 6) ELISA Kit
- Human IL-13(Interleukin 13) ELISA Kit
- Human IL-17A(Interleukin 17 A) ELISA Kit
- Human IL-19(Interleukin 19) ELISA Kit
- Human TNF-β(Tumor Necrosis Factor Beta) ELISA Kit
- Human IL-2(Interleukin 2) ELISA Kit
- Human IL-3(Interleukin 3) ELISA Kit
- Human IL-7(Interleukin 7) ELISA Kit
- Human IL-8(Interleukin 8) ELISA Kit
- Human IL-12(Interleukin 12) ELISA Kit
- Human IL-15(Interleukin 15) ELISA Kit
- Human IL-1α(Interleukin 1 Alpha) ELISA Kit
- Human IL-1β(Interleukin 1 Beta) ELISA Kit
- Human GM-CSF(Granulocyte Macrophage Colony Stimulating Factor) ELISA Kit
- Human IFN-γ(Interferon gamma) ELISA Kit
- Human G-CSF(Granulocyte Colony Stimulating Factor 3) ELISA Kit
- Human M-CSF(Macrophage Colony-Stimulating Factor) ELISA Kit
- Journal:
- Journal of Crohn's and Colitis
- Cited Date:
- 2019-01-25
- Product:
- Journal:
- Alimentary Pharmacology & Therapeutics
- Author:
- Department of Hepatology, Post Graduate Institute of Medical Education and Research, Chandigarh, India.
- Cited Date:
- 2024-08-23
- Product:
- Journal:
- International Journal of Molecular Sciences
- Cited Date:
- 2023-03-17
- Product:
- Journal:
- Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease
- Author:
- School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine, Guangzhou 510006, China
- Cited Date:
- 2024-02-18
- Product:
- Journal:
- Frontiers in Cellular and Infection Microbiology
- Author:
- Department of Neurology, the Affiliated Wuxi People’s Hospital of Nanjing Medical University, Wuxi People’s Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi, China
- Cited Date:
- 2023-07-28
- Product:
- Journal:
- International Journal of Molecular Sciences
- Author:
- Department of Biomedical Laboratory Sciences, School of Health Sciences, College of Medicine and Health Sciences, University of Rwanda, Kigali P.O. Box 3248, Rwanda
- Cited Date:
- 2023-08-11
- Product:
-
- Human IL-17 A/F(Interleukin 17A/F) ELISA Kit
- Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Human IL-6(Interleukin 6) ELISA Kit
- Human IL-13(Interleukin 13) ELISA Kit
- Human IgG(Immunoglobulin G) ELISA Kit
- Human IgM(Immunoglobulin M) ELISA Kit
- Human IL-4(Interleukin 4) ELISA Kit
- Human IL-10(Interleukin 10) ELISA Kit
- Human IFN-γ(Interferon gamma) ELISA Kit
- Journal:
- International Journal of Molecular Medicine
- Author:
- College of Emergency Trauma, Hainan Medical University, Haikou, Hainan 571199, P.R. China
- Species:
- supernatants
- Cited Date:
- 2024-06-14
- Product:
- Journal:
- Food & Function
- Cited Date:
- 2021-07-16
- Product:
- Journal:
- Biochemical Pharmacology
- Author:
- Department of Thoracic Surgery, Xingtai People's Hospital, Xingtai 054000 Hebei, PR China.
- Cited Date:
- 2025-02-07
- Product:
- Journal:
- Communications Biology
- Author:
- Department of Bioengineering, Faculty of Engineering, Ege University, Izmir, Türkiye.
- Sample:
- supernatant
- Cited Date:
- 2024-12-13
- Product:
- Journal:
- Frontiers in Medicine
- Cited Date:
- 2022-02-25
- Product:
- Journal:
- International Journal of Molecular Sciences
- Author:
- Department of Hospital Therapy No 1, I.M. Sechenov First Moscow State Medical University (Sechenov University), 119048 Moscow, Russia.
- Sample:
- plasma
- Cited Date:
- 2024-12-20
- Product:
-
- Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Human MMP14(Matrix metalloproteinase-14) ELISA Kit
- Human VEGF(Vascular Endothelial Cell Growth Factor) ELISA Kit
- Human MMP-1(Matrix Metalloproteinase 1) ELISA Kit
- Human MMP13(Matrix Metalloproteinase 13) ELISA Kit
- Human MMP-9(Matrix Metalloproteinase 9) ELISA Kit
- Journal:
- Stem Cell Reviews and Reports
- Author:
- Department of Bioengineering, Faculty of Engineering, Ege University, Izmir, Turkey
- Cited Date:
- 2023-11-17
- Product:
- Journal:
- Translational Oncology
- Author:
- Division of Hepatobiliary and Pancreatic Surgery, Department of Surgery, The First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, 310003, PR China.
- Sample:
- supernatants
- Cited Date:
- 2025-03-14
- Product:
- Journal:
- ACS Omega
- Author:
- Department of Neurology, the Affiliated Wuxi People's Hospital of Nanjing Medical University, Wuxi People's Hospital, Wuxi Medical Center, Nanjing Medical University, Wuxi 214023, China.
- Sample:
- plasma
- Cited Date:
- 2024-04-12
- Product:
- Journal:
- ACS Omega
- Author:
- Department of Pharmacognosy, Faculty of Pharmacy, Kafrelsheikh University, Kafrelsheikh 33516, Egypt
- Cited Date:
- 2023-10-20
- Product:
- Journal:
- Brain, Behavior, & Immunity - Health
- Author:
- Institute of Immunology, Faculty of Medicine, Comenius University in Bratislava, Odborarske namestie 14, 811 08, Bratislava, Slovakia
- Sample:
- serum
- Cited Date:
- 2025-04-18
- Product:
- Journal:
- Journal of Biochemical and Molecular Toxicology
- Cited Date:
- 2020-10-16
- Product:
- Journal:
- International Journal of Infectious Diseases
- Cited Date:
- 2020-03-10
- Product:
- Journal:
- Brain Sciences
- Author:
- Department of Neurosurgery & Brain and Nerve Research Laboratory, The First Affiliated Hospital of Soochow University, 188 Shizi Street, Suzhou 215006, China
- Cited Date:
- 2023-08-11
- Product:
- Journal:
- Current Alzheimer Research
- Cited Date:
- 2019-07-30
- Product:
- Journal:
- Medical Principles and Practice
- Author:
- Department of Gynecology and Obstetrics, Changchun University of Chinese Medicine Affiliated Hospital, Changchun 130000, Jilin, China
- Species:
- serum
- Cited Date:
- 2024-06-14
- Product:
- Journal:
- Translational Andrology and Urology
- Cited Date:
- 2021-09-24
- Product:
- Journal:
- Journal of Clinical Medicine
- Author:
- Department of Human Sciences and Promotion of the Quality of Life, San Raffaele Roma Open University, 00166 Roma, Italy.
- Sample:
- saliva
- Cited Date:
- 2025-04-18
- Product:
- Journal:
- Computational and Mathematical Methods in Medicine
- Cited Date:
- 2022-09-01
- Product:
- Journal:
- Current Issues in Molecular Biology
- Author:
- Department of Medico-Surgical Sciences and Biotechnologies, Sapienza University of Rome, 04100 Latina, Italy
- Sample:
- serum
- Cited Date:
- 2024-08-23
- Product:
- Journal:
- Journal of Applied Toxicology
- Author:
- Department of Intensive Care Unit, The Affiliated Cancer Hospital of Nanjing Medical University (Jiangsu Cancer Hospital, Jiangsu Institute of Cancer Research), Nanjing, Jiangsu, China.
- Cited Date:
- 2025-05-23
- Product:
- Journal:
- Photochemical & Photobiological Sciences
- Author:
- Department of Biology, Faculty of Sciences, Universidad Autónoma de Madrid, Madrid, Spain.
- Sample:
- culture media
- Cited Date:
- 2024-06-28
- Product:
- Journal:
- Molecular Biotechnology
- Author:
- Department of Oncology, Nantong First People's Hospital, the Second Affiliated Hospital of Nantong University, Nantong City, 226000, Jiangsu, China
- Cited Date:
- 2024-01-26
- Product:
- Journal:
- Nephrology
- Author:
- Department of Pharmacology Xingtai Medical College Xingtai Hebei China
- Cited Date:
- 2023-12-08
- Product:
- Journal:
- PeerJ
- Cited Date:
- 2021-08-12
- Product:
- Journal:
- Journal of Clinical Laboratory Analysis
- Cited Date:
- 2021-08-13
- Product:
- Journal:
- Molecular and Cellular Probes
- Author:
- Department of Oral Medicine and Prosthodontics, Hebei Medical University Third Hospital, 050000, China.
- Sample:
- Gingival Crevicular Fluid(GCF)
- Cited Date:
- 2025-02-21
- Product:
- Journal:
- Computational and Mathematical Methods in Medicine
- Cited Date:
- 2022-04-07
- Product:
- Journal:
- Advances in Rheumatology
- Cited Date:
- 2021-10-22
- Product:
- Journal:
- Toxicology Research
- Author:
- Gülhane Faculty of Pharmacy Department of Pharmaceutical Toxicology, University of Health Sciences Turkey, 06018 Ankara, Türkiye.
- Cited Date:
- 2024-11-15
- Product:
- Journal:
- American Journal of Translational Research
- Author:
- Department of General Surgery, The Second Affiliated Hospital of Jiaxing University, Jiaxing 314000, Zhejiang, China
- Cited Date:
- 2024-01-12
- Product:
- Journal:
- Canadian Respiratory Journal
- Author:
- Department of Pulmonology , Faculty of Medicine , Hatay Mustafa Kemal University , Antakya , Hatay, Türkiye
- Sample:
- serum
- Cited Date:
- 2025-06-20
- Product:
- Journal:
- Hereditas
- Author:
- Graduate School of Heilongjiang University of Traditional Chinese Medicine Harbin, Harbin, 150040, China.
- Sample:
- serum
- Cited Date:
- 2025-06-20
- Product:
- Journal:
- Indian Journal of Pediatrics
- Cited Date:
- 2021-10-08
- Product:
- Journal:
- The American Journal of the Medical Sciences
- Cited Date:
- 2019-12-05
- Product:
- Journal:
- Medical sicence monitor
- Cited Date:
- 2019-01-26
- Product:
- Journal:
- Molecular Biology Reports
- Cited Date:
- 2018-07-05
- Product:
- Journal:
- Cell Biochemistry and Biophysics
- Author:
- Department of Endodontics, Changsha Stomatological Hospital, No.389, Youyi Road, Tianxin District, Changsha, 410008, Hunan, China.
- Cited Date:
- 2024-10-08
- Product:
- Journal:
- American Journal of Translational Research
- Author:
- Department of Orthopedics, Lu’an Hospital of PKU HealthCare, Changzhi 046000, Shanxi, China
- Sample:
- serum
- Cited Date:
- 2024-12-13
- Product:
- Journal:
- Experimental Lung Research
- Author:
- Department of Respiratory and Critical Care Medicine, The Second Affiliated Hospital, Anhui Medical University, Hefei, China.
- Sample:
- supernatants
- Cited Date:
- 2024-07-26
- Product:
- Journal:
- Alternative Therapies in Health and Medicine
- Cited Date:
- 2022-01-20
- Product:
- Journal:
- Scandinavian Journal of Clinical and Laboratory Investigation
- Author:
- Faculty of Pharmacy, University of Belgrade, Belgrade, Serbia.
- Cited Date:
- 2024-11-15
- Product:
-
- Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Human IL-6(Interleukin 6) ELISA Kit
- Human suPAR(soluble urokinase-type plasminogen activator receptor) ELISA Kit
- Human SAA(Serum Amyloid A) ELISA Kit
- Human PTX3(Pentraxin 3) ELISA Kit
- Human IL-1β(Interleukin 1 Beta) ELISA Kit
- Human MPO(Myeloperoxidase) ELISA Kit
- Journal:
- International Food Research Journal
- Cited Date:
- 2022-03-11
- Product:
- Journal:
- Nigerian Journal of Clinical Practice
- Author:
- Department of Restorative Dentistry, Faculty of Dentistry, Gaziantep University, ?ehitkamil, Gaziantep, Turkey
- Cited Date:
- 2024-02-18
- Product:
- Journal:
- Journal of Immunoassay and Immunochemistry
- Cited Date:
- 2019-07-31
- Product:
- Journal:
- Pharmacognosy Journal
- Cited Date:
- 2023-01-29
- Product:
- Journal:
- Research Square
- Cited Date:
- 2020-06-30
- Product:
- Journal:
- Advances in Social Science, Education and Humanities Research
- Cited Date:
- 2021-03-18
- Product:
- Journal:
- Exerc Sci.
- Cited Date:
- 2016-11-30
- Product:
- Journal:
- Biomedical and Pharmacology Journal
- Author:
- Ibn El Jazzar Faculty of Medicine, University of Sousse, Tunisia
- Cited Date:
- 2024-01-19
- Product:
- Journal:
- Ukrainian Journal of Radiology and Oncology
- Cited Date:
- 2023-02-09
- Product:
- Journal:
- Preprints
- Author:
- Department of Hospital Therapy No 1, I.M. Sechenov First Moscow State Medical University (Sechenov University), 119991, Moscow, Russia
- Sample:
- plasma
- Cited Date:
- 2024-11-29
- Product:
-
- Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Human MMP14(Matrix metalloproteinase-14) ELISA Kit
- Human VEGF(Vascular Endothelial Cell Growth Factor) ELISA Kit
- Human MMP-1(Matrix Metalloproteinase 1) ELISA Kit
- Human MMP13(Matrix Metalloproteinase 13) ELISA Kit
- Human MMP-9(Matrix Metalloproteinase 9) ELISA Kit
- Journal:
- Journal of Dentomaxillofacial Science
- Cited Date:
- 2020-09-01
- Product:
- Journal:
- Thi-Qar Medical Journal
- Author:
- Ibn El Jazzar Faculty of Medicine, University of Sousse, Tunisia.
- Sample:
- serum
- Cited Date:
- 2024-05-06
- Product:
- Journal:
- Research Square
- Author:
- Nizam's Institute of Medical Sciences
- Cited Date:
- 2023-06-16
- Product:
-
- Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Human IL-6(Interleukin 6) ELISA Kit
- Human FGF2(Heparin-binding growth factor 2) ELISA Kit
- Human VEGF(Vascular Endothelial Cell Growth Factor) ELISA Kit
- Human IL-4(Interleukin 4) ELISA Kit
- Human IL-8(Interleukin 8) ELISA Kit
- Human IL-10(Interleukin 10) ELISA Kit
- Human IL-1β(Interleukin 1 Beta) ELISA Kit
- Human IFN-γ(Interferon gamma) ELISA Kit
- Journal:
- Egyptian Journal of Immunology
- Cited Date:
- 2021-11-25
- Product:
- Journal:
- The Medical Journal of Cairo University
- Cited Date:
- 2019-09-29
- Product:
- Journal:
- Indian Journal of Endocrinology and Metabolism
- Cited Date:
- 2023-03-16
- Product:
- Journal:
- Research Square
- Cited Date:
- 2021-05-28
- Product:
- Journal:
- Research Square
- Author:
- Gaziantep University
- Cited Date:
- 2023-11-03
- Product:
- Journal:
- Gastroenterology
- Author:
- State Institution “Institute of Gastroenterology of the National Academy of Medical Sciences of Ukraine”, Dnipro, Ukraine
- Cited Date:
- 2023-06-30
- Product:
- Journal:
- Exploration of Cardiology
- Author:
- Clatterbridge Centre for Oncology NHS Foundation Trust, L7 8YA Liverpool, UK.
- Sample:
- plasma or serum
- Cited Date:
- 2024-09-27
- Product:
- Journal:
- Dental Hypotheses
- Cited Date:
- 2018-07-13
- Product:
- Journal:
- Archives of Pharmaceutical Sciences Ain Shams University
- Cited Date:
- 2021-06-18
- Product:
- Journal:
- Archives of Pharmaceutical Sciences Ain Shams University
- Cited Date:
- 2021-06-18
- Product:
- Journal:
- Sana'a University Journal of Medical and Health Sciences
- Author:
- Orthodontics, Pedodontics and Prevention Department Faculty of Dentistry, Sana'a University, Yemen
- Cited Date:
- 2023-11-17
- Product:
- Journal:
- Journal of Musculoskeletal and Neuronal Interactions
- Author:
- Cukurova University Faculty of Medicine, Department of Biophysics Adana, Turkiye.
- Sample:
- cartilage samples
- Cited Date:
- 2024-08-02
- Product:
- Journal:
- medRxiv
- Cited Date:
- 2021-07-29
- Product:
-
- Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- DA(Dopamine) ELISA Kit
- Human IL-6(Interleukin 6) ELISA Kit
- Human 5-hydroxytryptamine ELISA Kit
- Human IL-17A(Interleukin 17 A) ELISA Kit
- Human IL-23(Interleukin 23) ELISA Kit
- Human IL-10(Interleukin 10) ELISA Kit
- Human IL-1β(Interleukin 1 Beta) ELISA Kit
- Journal:
- OSFPREPRINTS
- Cited Date:
- 2022-02-10
- Product:
-
- Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- DA(Dopamine) ELISA Kit
- Human IL-6(Interleukin 6) ELISA Kit
- ST/5-HT(5-hydroxytryptamine) ELISA Kit
- Human IL-17A(Interleukin 17 A) ELISA Kit
- Human IL-23(Interleukin 23) ELISA Kit
- Human IL-10(Interleukin 10) ELISA Kit
- Human IL-1β(Interleukin 1 Beta) ELISA Kit
- Journal:
- Journal of Ayurveda and Integrative Medicine
- Cited Date:
- 2021-08-12
- Product:
- Journal:
- Journal of Clinical Practice and Research
- Cited Date:
- 2023-04-21
- Product:
- Journal:
- Trends in Sciences
- Author:
- Department of Nursing Pharmacology, STIKES Panakkukang, Makassar, South Sulawesi 90231, Indonesia
- Sample:
- culture supernatants
- Cited Date:
- 2024-03-15
- Product:
- Journal:
- SSRN
- Author:
- State Key Laboratory of Oral Diseases, National Center for Stomatology, National Clinical Research Center for Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu 610041, P. R. China
- Sample:
- supernatant
- Cited Date:
- 2025-04-11
- Product:
- Journal:
- Malaysian Journal of Medicine and Health Sciences
- Cited Date:
- 2022-12-29
- Product:
- Journal:
- Journal of Exercise Nutrition & Biochemistry
- Cited Date:
- 2019-03-31
- Product:
- Journal:
- Korean Journal of Food Science and Technology
- Cited Date:
- 2019-04-30
- Product:
-
- Human TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- PG(Progesterone) ELISA Kit
- Human ADP(Adiponectin) ELISA Kit
- Human SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Human APOA1(Apolipoprotein A-I) ELISA Kit
- Human HS-INS(High sensitive Insulin) Accquant ELISA Kit
- Human LEP(Leptin) ELISA Kit
- Human ApoB100(Apolipoprotein B100) ELISA Kit
- Journal:
- Heliyon
- Cited Date:
- 2021-02-19
- Product:
- Journal:
- Medical Records
- Cited Date:
- 2021-09-10
- Product:
- Journal:
- Research Square
- Cited Date:
- 2023-01-28
- Product:
What’s the plate size in FineTest® ELISA Kits?
The ELISA plate follows the standard size of microplate: 127.64 mm x 85.60 mm x 14.22 mm(L x W x H).
How about the shelf life and stability of FineTest® ELISA Kits?
Valid for 12 months since the production date. For the shelf life of specific batch number, please check the label printed on the kit. Before delivery, all FineTest® ELISA Kits have been subject to strict quality test.
Which cloned antibodies for FineTest® ELISA Kits are used?
These information is proprietary. Please contact us to learn more about clonality (polyclonality or monoclonality) and host species.
Can I mix reagents from different batches of FineTest® ELISA Kits?
Not suggested. ELISA reagents are optimized for specific batch.
Can FineTest® ELISA Kits be used partially?
Yes. The ELISA plate is dismounted. Enough component volumes are offered by 96T ELISA kit, supporting two groups of standard curve.
How long can the diluted lyophilized standard be stored for continual use?
Used up within 12h.
Can standard curve be extended to any direction?
FineTest® can't support validation of standard concentration outside of standard curve. Ranges of standard curve have been validated among many batches and experimenters, showing stable and accurate performance. The lowest standard concentration is the minimized range for reliable detection results. Adding higher or lower concentration of standard may cause inconsistent signal or false positive.
Why does detection for serum/plasma sample by FineTest® ELISA Kits require for 1/2 dilution?
Matrix components in serum/plasma can affect detection results. Blocking components in sample dilution buffer can decrease or remove the interference. The dilution can reduce the matrix difference between sample and standard to get better accuracy.
What’s the half-life of protein in serum/plasma/cell culture supernatant?
FineTest® can't determine the half-life of protein in the sample(e.g. serum, plasma or cell culture supernatant). Usually, it's suggested to detect prepared sample immediately or aliquot sample to refrigerate in a disposable container. Avoid freeze-thaw cycle to prevent protein degradation.
What's the expected concentration for particularly analyzing my sample?
Due to the specificity of each sample, it's hard to forecast and depend on sample preparation as well as analytical characteristics. Please contact us to get detection data for reference.
How many samples can FineTest® ELISA Kits detect?
It depends on whether duplicate assay for your sample is required. Such as, you can detect 80 samples without duplication or 40 samples.
Why is sample loading conducted after equilibrating all kits’ components and samples to room temperature?
Temperature is the important factor for ELISA binding reaction. The reaction of samples at a consistent temperature requires for equilibrating all reagents to room temperature before the assay, including tested samples. Avoid the inaccurate ELISA assay results caused by temperature differences.
Why is duplicate assay suggested for ELISA assay?
To get more accurate assay results, it's strongly suggested to conduct duplicate assay for the standard and sample. Duplicate assay can calculate average value to ensure more accurate assay results and solve drift caused by misoperation during the assay. Calculation of CV evaluates experimental operation and precision of the ELISA kit.
How to get the standard curve with excellent linearity?
Follow the suggested method in the manual to store the standard. Before dissolving the standard, transient centrifugation is required to completely collect the powder. Confirm the fully dissolution and mixing of the standard(about 10min). Then, conduct steps of series dilution. Ensure the fully mixing and accurate pipetting in each step. Properly stop the staining. Choose suitable fitting equation to plot the standard curve.
Can ELISA plates be stacked together for incubation?
To keep the consistent environmental condition of all plates, stacking is not suggested during incubation.
Why is polypropylene tube used for standard dilution in some analyses?
Some proteins or analytes can bind with glass or polystyrene. However, they are not easy to bind with polypropylene tube.
When to stop ELISA reaction?
ELISA assay finally requires for enzyme-catalyzed substrate to complete the staining reaction. Stopping the reaction at the best time is the important factor for successful ELISA assay. When the blue complex appears after adding TMB substrate for 12min, read the O.D. absorbance at 620nm. The staining can be stopped, when the OD value of the darkest color at 620nm is between 0.8 - 0.9. The relevant OD value at 450nm for stopping the staining is between 2.0 - 2.5. If the OD value at 620nm is lower than 0.8, the staining duration can be properly extended.
Why is dual wavelength selected for reading in a microplate reader? What's the purpose of wavelength correction?
Dual wavelength for reading in ELISA assay mainly aims to remove non-specific background interference, and improve accuracy and reliability of the detection. FineTest® usually suggests to set the corrected wavelength as 570nm or 630nm in a microplate reader. The OD value may be higher when directly reading at 450nm. However, the accuracy is lower.
Why is 4 - pl curve fitting is required for generating standard curve?
FineTest® recommends to use ELISA curve fitting software and validate ELISA kit with 4 - pl curve fitting. 4 - pl curve fitting mainly aims to accurately describe the nonlinear dynamic characteristics of antigen - antibody binding. Thus, the quantitative accuracy of low or high concentration of sample is improved. Experimental errors are decreased. The reliability and reproducibility of assay results are ensured.
If sample OD value is higher than OD value of the highest point on the standard curve, what's the suggested solution?
Dilute the sample with dilution buffer and conduct the detection again. Ensure the detected value of the sample falls in the range of standard curve.
Are there any rewards to publish papers using FineTest® ELISA Kits?
If you published paper using FineTest® ELISA Kits, you will obtain US$150-US$550 coupon, please contact us for more details.