Products
Mouse Hmgb1(High mobility group protein B1) ELISA Kit
This kit is based on Double antibody-Sandwich ELISA detection method and takes 4h assay time. The microplate provided in this kit has been precoated with anti Hmgb1 antibody. Add standard and properly diluted sample into relevant well respectively. After incubation, wash unbound components. Add biotinylated detection antibody. Then, it binds with Hmgb1 bound to precoated antibody. Wash unbound components and add HRP-Streptavidin Conjugate (SABC). Wash unbound components again and add TMB substrate solution. Then, TMB was catalyzed by HRP to produce a blue color product that turned yellow after adding a stop solution. Read the O.D. absorbance at 450nm in a microplate reader. Calculate the concentration of Hmgb1 in the sample by plotting standard curve. The concentration of the target substance is proportional to the OD450 value.
- Catalogue No.:
- EM0382
- Alias:
- High mobility group protein B1 ELISA Kit, High mobility group protein 1 ELISA Kit, HMG-1 ELISA Kit, HMGB1 ELISA Kit, HMG1 ELISA Kit
- Species:
- Mouse
- Range:
- 15.625-1000pg/ml
- Sensitivity:
- 9.375pg/ml
| Size | Price |
|---|---|
| 96T | Inquiry |
| 48T | Inquiry |
- SPECIFICATIONS
- CITATIONS
- FIGURES
- CONDITIONS
- FAQS
- Product Name
- Mouse Hmgb1(High mobility group protein B1) ELISA Kit
- Alias
- High mobility group protein B1 ELISA Kit, High mobility group protein 1 ELISA Kit, HMG-1 ELISA Kit, HMGB1 ELISA Kit, HMG1 ELISA Kit
- Catalogue No.
- EM0382
- Size
- 48T/96T
- Species
- Mouse
- UniProt ID
- P63158
- Sample Type
- Serum, Plasma, Cell Culture Supernatant, cell or tissue lysate, Other liquid samples
- Detection Method
- Sandwich ELISA, Double Antibody
- Detection Wavelength
- OD450
- Reaction Duration
- 4 hours
- Range
- 15.625-1000pg/ml
- Sensitivity
- 9.375pg/ml
- Storage
- 2-8°C(Sealed), Don't cryopreserve.
- Specificity
- Specifically binds with Hmgb1 , no obvious cross reaction with other analogues.
- ELISA Kit Components
Kit Components Item Size(48T) Size(96T) Storage Condition for Opened Kit E001 ELISA Microplate(Dismountable) 8×6 8×12 Put the rest strips into a sealed foil bag with the desiccant. Stored for 1 month at 2-8°C; Stored for 6 month at -20°C E002 Lyophilized Standard 1vial 2vial Put the rest standards into a desiccant bag. Stored for 1 month at 2-8°C; Stored for 6 month at -20°C E003 Biotin-labeled Antibody(Concentrated, 100X) 60ul 120ul 2-8°C (Avoid Direct Light) E034 HRP-Streptavidin Conjugate(SABC, 100X) 60ul 120ul E024 TMB Substrate 5ml 10ml E039 Sample Dilution Buffer 10ml 20ml 2-8°C E040 Antibody Dilution Buffer 5ml 10ml E049 SABC Dilution Buffer 5ml 10ml E026 Stop Solution 5ml 10ml E038 Wash Buffer(Concentrated, 25X) 15ml 30ml E006 Plate Sealer 3 pieces 5 pieces E007 Product Description 1 copy 1 copy - Required Instruments and Reagents
-
- Microplate reader (wavelength: 450nm)
- 37°C incubator (CO2 incubator for cell culture is not recommenced.)
- Automated plate washer or multi-channel pipette/5ml pipettor (for manual washing purpose)
- Precision single (0.5-10μL, 5-50μL, 20-200μL, 200-1000μL) and multi-channel pipette with disposable tips(Calibration is required before use.)
- Sterile tubes and Eppendorf tubes with disposable tips
- Absorbent paper and loading slot
- Deionized or distilled water
- Assay Procedure Summary
-
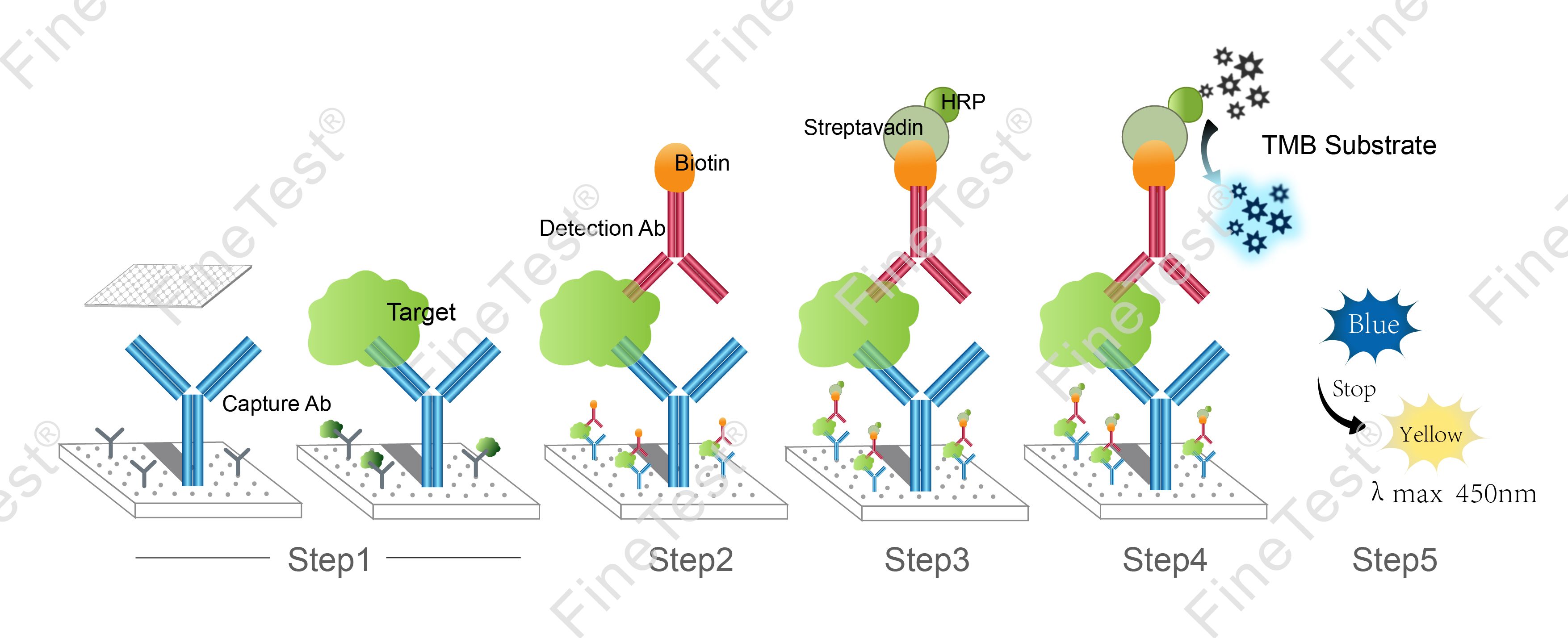
- Step 1: Add 100ul standard or sample into each well, seal the plate and statically incubate for 90 minutes at 37°C.
- Washing: Wash the plate twice without immersing.
- Step 2: Add 100ul biotin-antibody working solution, seal the plate and statically incubate for 60 minutes at 37°C.
- Washing: Wash the plate three times and immerse for 1min each time.
- Step 3: Add 100ul HRP-Streptavidin Conjugate (SABC) working solution, seal the plate and statically incubate for 30 minutes at 37°C.
- Washing: Wash the plate five times and immerse for 1min each time.
- Step 4: Add 90ul TMB substrate solution, seal the plate and statically incubate for 10-20 minutes at 37°C. (Accurate TMB visualization control is required.)
- Step 5: Add 50ul stop solution. Read at 450nm immediately and calculate.
- Standard Curve
-
This product is detected by QC department and meets performance required in the manual. (Laboratory Humidity: 20%-60%; Temperature: 18°C -25°C; Equilibrate TMB substrate to 37°C before staining. After adding into the ELISA wells, incubate for 15min at 37°C in dark.)
Due to different assay environments and operations, assay data below and standard curve are provided for reference. Experimenters should establish standard curve according to their own assay.
STD.(pg/ml) OD-1 OD-2 Average 0 0.086 0.091 0.088 15.625 0.134 0.141 0.137 31.25 0.182 0.192 0.186 62.5 0.308 0.323 0.314 125 0.514 0.54 0.524 250 0.823 0.865 0.84 500 1.372 1.442 1.4 1000 2.052 2.157 2.094 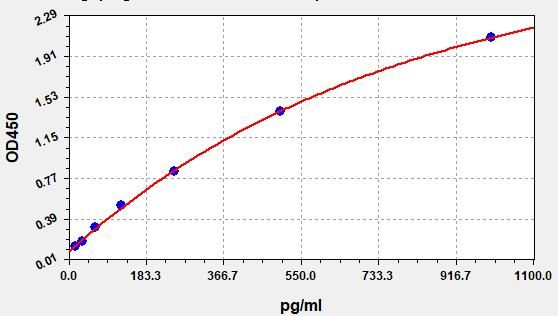
- Recovery
-
Add a certain amount of Hmgb1 into the sample. Calculate the recovery by comparing the measured value with the expected amount of Hmgb1 in the sample.
Sample Type Recovery Range(%) Average(%) serum(n=10) 95-105 97 EDTA plasma(n=10) 86-101 95 Heparin plasma(n=10) 92-103 93 - Linearity
-
Dilute the sample with a certain amount of Hmgb1 at 1:2, 1:4 and 1:8 to get the recovery range.
Sample Type 1:2 1:4 1:8 serum(n=10) 92-100% 88-105% 89-105% EDTA plasma(n=10) 86-99% 82-90% 87-96% Heparin plasma(n=10) 84-100% 87-99% 80-104% - Precision(%)
-
Intra-assay Precision: samples with low, medium and high concentration are tested 20 times on the same plate.
Inter-assay Precision: samples with low, medium and high concentration are tested 20 times on three different plates.
Item Intra-assay Precision Inter-assay Precision Sample 1 2 3 1 2 3 n 20 20 20 20 20 20 Mean (pg/ml) 32.54 126.7 480 32.77 129.7 482 Standard deviation 1.7 6.31 32.06 1.58 6.39 28.49 CV(%) 5.23 4.98 6.68 4.82 4.93 5.91 - Stability
-
Perform the stability test for the sealed kit at 37°C and 2-8°C and get relevant data.
ELISA kit(n=5) 37°C for 1 month 2-8°C for 6 months Average(%) 80 95-100
- Journal:
- Immunity
- Author:
- Department of Neurology, Tianjin Neurological Institute, Tianjin Institute of Immunology, State Key Laboratory of Experimental Hematology, International Joint Laboratory of Ocular Diseases, Ministry of Education, Haihe Laboratory of Cell Ecosystem, Labora
- Cited Date:
- 2024-08-02
- Product:
-
- Mouse Dll3(Delta-like protein 3)ELISA Kit
- Mouse Dll4(Delta-like protein 4)ELISA Kit
- Human JAG1(Protein jagged-1)ELISA Kit
- Mouse Jag2(Protein jagged-2)ELISA Kit
- Mouse Dll1(Delta-like protein 1)ELISA Kit
- Mouse Jag1(Protein jagged-1)ELISA Kit
- Mouse Hmgb1(High mobility group protein B1) ELISA Kit
- Human DLL1(Delta-like protein 1) ELISA Kit
- Journal:
- Kidney International
- Author:
- Cardiovascular Research Center, Shantou University Medical College, Shantou, China
- Cited Date:
- 2022-09-23
- Product:
- Journal:
- Nature Communications
- Author:
- School of Chemistry, Chemical Engineering and Biotechnology, Nanyang Technological University, Singapore, 637371, Singapore
- Cited Date:
- 2023-06-02
- Product:
- Journal:
- ACS Nano
- Author:
- School of Medicine, Chongqing University, Chongqing 400044, China.
- Cited Date:
- 2024-05-31
- Product:
- Journal:
- ACS Nano
- Author:
- National Engineering Research Center for Nanomedicine, College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan 430074, China.
- Cited Date:
- 2025-04-25
- Product:
- Journal:
- Autophagy
- Author:
- Department of Neurology, Mental and Neurological Disease Research Center, the Third Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
- Cited Date:
- 2023-10-13
- Product:
- Journal:
- Biomaterials
- Author:
- Department of Nanomedicine and Biopharmaceuticals, College of Life Science and Technology, Huazhong University of Science and Technology, Wuhan, 430074, P. R. China
- Cited Date:
- 2024-08-23
- Product:
- Journal:
- Science Advances
- Author:
- State Key Laboratory of Integrated Optoelectronics, JLU Region, College of Electronic Science and Engineering, Jilin University, Changchun 130012, China.
- Sample:
- 4T1 cells
- Cited Date:
- 2025-06-06
- Product:
- Journal:
- Molecular Therapy
- Cited Date:
- 2021-12-24
- Product:
- Journal:
- Journal of Controlled Release
- Author:
- The Fifth Affiliated Hospital, Key Laboratory of Molecular Target & Clinical Pharmacology and the State Key Laboratory of Respiratory Disease, School of Pharmaceutical Sciences, Guangzhou Medical University, Guangzhou 511436, PR China.
- Cited Date:
- 2024-08-30
- Product:
- Journal:
- Nano Research
- Cited Date:
- 2022-07-08
- Product:
- Journal:
- Journal for ImmunoTherapy of Cancer
- Cited Date:
- 2020-03-01
- Product:
- Journal:
- ACS Applied Materials & Interfaces
- Author:
- College of Pharmaceutical Science, Zhejiang University of Technology, Hangzhou 310014, China.
- Cited Date:
- 2024-10-18
- Product:
- Journal:
- International Journal of Nanomedicine
- Author:
- State Key Laboratory of Vaccines for Infectious Diseases, XiangAn Biomedicine Laboratory, School of Public Health, Xiamen University, Xiamen, People’s Republic of China.
- Sample:
- 4T1 cells
- Cited Date:
- 2025-04-25
- Product:
- Journal:
- iScience
- Cited Date:
- 2022-03-04
- Product:
- Journal:
- Journal of Ethnopharmacology
- Author:
- Department of Microbiology, College of Medicine, Chung-Ang University, 84 Heukseok-ro, Dongjak-gu, Seoul, 06974, Republic of Korea
- Cited Date:
- 2023-06-23
- Product:
- Journal:
- Naunyn-Schmiedeberg's Archives of Pharmacology
- Author:
- Department of Pharmaceutics, Faculty of Pharmacy, Helwan University, Cairo, Egypt
- Cited Date:
- 2024-01-26
- Product:
- Journal:
- Molecular Medicine Reports
- Author:
- Department of Cardiovascular Medicine, Zhongda Hospital Southeast University, Nanjing, Jiangsu 210009, P.R. China
- Cited Date:
- 2024-01-05
- Product:
- Journal:
- BMC Complementary Medicine and Therapies
- Author:
- Department of Medical Services and Techniques, Vocational School of Health Services, Recep Tayyip Erdogan University, Rize, 53100, Türkiye, Turkey.
- Sample:
- serum
- Cited Date:
- 2025-06-13
- Product:
-
- Mouse ATF6 (Activating transcription factor 6) ELISA Kit
- Mouse GCLC(Glutamate-cysteine ligase catalytic subunit) ELISA Kit
- Mouse T(Testosterone) ELISA Kit
- Mouse CHOP(C/EBP Homologous Protein)ELISA Kit
- Mouse NRF2(NF-E2-related factor 2) ELISA Kit
- Mouse HSPA5(78 kDa glucose-regulated protein) ELISA Kit
- Mouse NF-κB p65(Nuclear Factor-κB p65) ELISA Kit
- Mouse INHB(Inhibin B) ELISA Kit
- Mouse HO-1(Heme Oxygenase 1) ELISA Kit
- Mouse SOD1(Superoxide Dismutase [Cu-Zn]) ELISA Kit
- Mouse Hmgb1(High mobility group protein B1) ELISA Kit
- Mouse Casp3(Caspase-3) ELISA Kit
- Mouse IL-6(Interleukin 6) ELISA Kit
- Mouse MPO(Myeloperoxidase) ELISA Kit
- Journal:
- Clinics
- Author:
- Department of Orthopedics, Jianhu People's Hospital, Yancheng, Jiangsu, China
- Cited Date:
- 2023-07-28
- Product:
- Journal:
- SSRN
- Author:
- Research Center for Drug and Vaccine Development, National Institute of Infectious Diseases; Tokyo, 162-8640, Japan.
- Sample:
- plasma
- Cited Date:
- 2025-03-07
- Product:
- Journal:
- Research Square
- Author:
- Houston Methodist Research Institute
- Sample:
- serum
- Cited Date:
- 2024-10-25
- Product:
What’s the plate size in FineTest® ELISA Kits?
The ELISA plate follows the standard size of microplate: 127.64 mm x 85.60 mm x 14.22 mm(L x W x H).
How about the shelf life and stability of FineTest® ELISA Kits?
Valid for 12 months since the production date. For the shelf life of specific batch number, please check the label printed on the kit. Before delivery, all FineTest® ELISA Kits have been subject to strict quality test.
Which cloned antibodies for FineTest® ELISA Kits are used?
These information is proprietary. Please contact us to learn more about clonality (polyclonality or monoclonality) and host species.
Can I mix reagents from different batches of FineTest® ELISA Kits?
Not suggested. ELISA reagents are optimized for specific batch.
Can FineTest® ELISA Kits be used partially?
Yes. The ELISA plate is dismounted. Enough component volumes are offered by 96T ELISA kit, supporting two groups of standard curve.
How long can the diluted lyophilized standard be stored for continual use?
Used up within 12h.
Can standard curve be extended to any direction?
FineTest® can't support validation of standard concentration outside of standard curve. Ranges of standard curve have been validated among many batches and experimenters, showing stable and accurate performance. The lowest standard concentration is the minimized range for reliable detection results. Adding higher or lower concentration of standard may cause inconsistent signal or false positive.
Why does detection for serum/plasma sample by FineTest® ELISA Kits require for 1/2 dilution?
Matrix components in serum/plasma can affect detection results. Blocking components in sample dilution buffer can decrease or remove the interference. The dilution can reduce the matrix difference between sample and standard to get better accuracy.
What’s the half-life of protein in serum/plasma/cell culture supernatant?
FineTest® can't determine the half-life of protein in the sample(e.g. serum, plasma or cell culture supernatant). Usually, it's suggested to detect prepared sample immediately or aliquot sample to refrigerate in a disposable container. Avoid freeze-thaw cycle to prevent protein degradation.
What's the expected concentration for particularly analyzing my sample?
Due to the specificity of each sample, it's hard to forecast and depend on sample preparation as well as analytical characteristics. Please contact us to get detection data for reference.
How many samples can FineTest® ELISA Kits detect?
It depends on whether duplicate assay for your sample is required. Such as, you can detect 80 samples without duplication or 40 samples.
Why is sample loading conducted after equilibrating all kits’ components and samples to room temperature?
Temperature is the important factor for ELISA binding reaction. The reaction of samples at a consistent temperature requires for equilibrating all reagents to room temperature before the assay, including tested samples. Avoid the inaccurate ELISA assay results caused by temperature differences.
Why is duplicate assay suggested for ELISA assay?
To get more accurate assay results, it's strongly suggested to conduct duplicate assay for the standard and sample. Duplicate assay can calculate average value to ensure more accurate assay results and solve drift caused by misoperation during the assay. Calculation of CV evaluates experimental operation and precision of the ELISA kit.
How to get the standard curve with excellent linearity?
Follow the suggested method in the manual to store the standard. Before dissolving the standard, transient centrifugation is required to completely collect the powder. Confirm the fully dissolution and mixing of the standard(about 10min). Then, conduct steps of series dilution. Ensure the fully mixing and accurate pipetting in each step. Properly stop the staining. Choose suitable fitting equation to plot the standard curve.
Can ELISA plates be stacked together for incubation?
To keep the consistent environmental condition of all plates, stacking is not suggested during incubation.
Why is polypropylene tube used for standard dilution in some analyses?
Some proteins or analytes can bind with glass or polystyrene. However, they are not easy to bind with polypropylene tube.
When to stop ELISA reaction?
ELISA assay finally requires for enzyme-catalyzed substrate to complete the staining reaction. Stopping the reaction at the best time is the important factor for successful ELISA assay. When the blue complex appears after adding TMB substrate for 12min, read the O.D. absorbance at 620nm. The staining can be stopped, when the OD value of the darkest color at 620nm is between 0.8 - 0.9. The relevant OD value at 450nm for stopping the staining is between 2.0 - 2.5. If the OD value at 620nm is lower than 0.8, the staining duration can be properly extended.
Why is dual wavelength selected for reading in a microplate reader? What's the purpose of wavelength correction?
Dual wavelength for reading in ELISA assay mainly aims to remove non-specific background interference, and improve accuracy and reliability of the detection. FineTest® usually suggests to set the corrected wavelength as 570nm or 630nm in a microplate reader. The OD value may be higher when directly reading at 450nm. However, the accuracy is lower.
Why is 4 - pl curve fitting is required for generating standard curve?
FineTest® recommends to use ELISA curve fitting software and validate ELISA kit with 4 - pl curve fitting. 4 - pl curve fitting mainly aims to accurately describe the nonlinear dynamic characteristics of antigen - antibody binding. Thus, the quantitative accuracy of low or high concentration of sample is improved. Experimental errors are decreased. The reliability and reproducibility of assay results are ensured.
If sample OD value is higher than OD value of the highest point on the standard curve, what's the suggested solution?
Dilute the sample with dilution buffer and conduct the detection again. Ensure the detected value of the sample falls in the range of standard curve.
Are there any rewards to publish papers using FineTest® ELISA Kits?
If you published paper using FineTest® ELISA Kits, you will obtain US$150-US$550 coupon, please contact us for more details.