Abstract: Systemic lupus erythematosus(SLE) is an autoimmune diseases featured with slow onset, insidious occurrence, clinical manifestations. SLE is involved in many system and organ autoimmune diseases and has an effect on skin, serosa, joints, kidney and central nervous system etc. Cellular and humoral immune dysfunctions produce many autoantibodies.
Keywords: Systemic Lupus Erythematosus, Autoimmune Disease, Inflammation
1. Causes of SLE Disease
Main clinical features of SLE disease are edematous lupus and rash across the bridge of the nose and cheeks.
Many autoantibodies in the patient's body affect humoral and cellular immunity. Complement system also changes. SLE disease may be mainly caused by formation of immune complexes. The exact etiology remains unclear. This disease is recurrent and chronic, and often happens in young women. It's not easy to completely cure the disease. However, most patient's condition can be better controlled via effective treatment.
The interreaction among estrogen level, hereditary and environmental factors results in the decrease of T lymphocytes and T suppressor cells, over hyperplasia of B cell, and various autoantibodies which bind with relevant autoantigens to form immune complexes sediment in skin, joints, small vessels and glomerulus etc. Complement helps to cause acute and chronic inflammation, and tissue necrosis(e.g. lupus nephritis). Alternatively, antibodies directly have an effect on histocyte antigens to cause cell damage-induced multisystem injury(e.g. specific antigens of erythrocyte, lymphocyte and platelet wall bind with relevant antibodies, resulting in hemolytic anemia, lymphopenia and thrombocytopenia respectively).
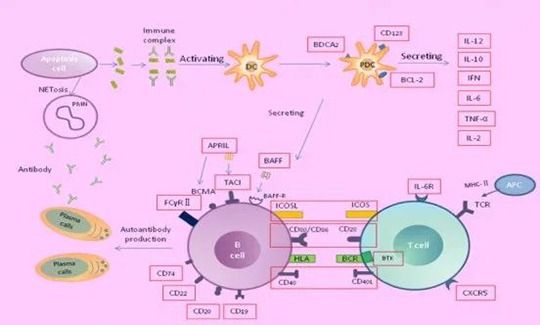
2. Systemic Lupus Diagnosis
2.1. Immunologic Detection
Various autoantibodies are found in the blood. Antinuclear antibody(ANA) is nearly 100% positive during active disease. Positive may still appear when changing method for detecting negative. The negative ANA can’t completely exclude this disease. The comprehensive analysis of data from clinical and other laboratory detection is required. Anti-ds DNA antibody has a higher specific diagnosis, but the positive rate is lower(40-75%, which is closely related to disease activity and kidney damage). Antibody titer decreases with the relieved situation. Anti-Sm antibody is positive in about 30% SLE disease. Due to the high specificity, it's the specific antibody of this disease. For atypical, mild or early cases, the diagnosis following SLE criteria may be undefined but anti-Sm antibody is positive, the diagnosis with other symptoms can be defined.
Lupus cells are neutrophil phagocytosis-induced cells produced by the binding between nuclear material released from damaged leucocyte and antinuclear antibody(ANA). The positive rate is about 60%. C4, C3 and CH50 obviously decrease in serum complement of active cases. When combined with lupus nephritis, immune complexes in blood circulation increase.
Besides autoantibodies above, other autoantibodies in SLE patients' blood can be detected.
2.2. Immunopathology Detection
Immunofluorescence research on living tissue sections of renopuncture shows main immunoglobulins and complements(IgG, IgM) deposit in the SLE kidney. Three kinds of deposition include mesangial, subendothelial and subepithelial. The deposition along the glomerular basement membrane presents granular distribution. Mesangial deposition is irregular, granular or uniform-chain between capillary loops. Immune complex(IC) is found in kidney tubular basement membrane and mesenchyme of 20-50% lupus nephritis patients. Clinical features and abnormality of urine and renal biopsy are not completely consistent. Lupus band test uses immunofluorescence method to check the junction of dermis and epidermis of patients' skin and find immunoglobulin(IgG, IgM) and complement deposition are arranged in line, granula or globularity, forming yellow-green fluorescent band. The positive rate for normal skin exposure area and skin lesions area is 50-70% and over 90% respectively. Positive lupus band on he non-exposure area shows the severe disease with possible nephritis, hypocomplementemia and high DNA antibody level.
3. Recommended ELISA Kits
|
Cat.No |
Product Name |
|
Human BAFF(B-Cell Activating Factor) ELISA Kit |
|
|
BAFF: BAFF or BLyS is a B cell survival factor, supporting auto-reactive B cells and preventing the loss. The expression of BAFF is closely related to autoimmunity. BAFF is commonly expressed in SLE and related to pathogenesis. It takes en effect in renal involvement. Belimumab is a biological inhibitor for BAFF, and is the first biological agent approved for the SLE treatment. |
|
|
Human Bcl-2(B-cell Leukemia/Lymphoma 2) ELISA Kit |
|
|
Bcl-2: SLE is a lymphatic hyperreactivity disease. The expression of bcl-2 in SLE patients' lymphocyte, and possibly interfere in programmed cell death, resulting in lymphatic hyperreactivity. |
|
|
Human CD19(Cluster of Differentiation 19) ELISA Kit |
|
|
CD19: SLE is a systemic autoimmune disease. B cell is abnormally activated to produce autoantibodies. CD19 and CD81 in SLE patients' peripheral blood obviously decrease, which are related to disease activity and potential targets for diagnosis and monitoring of SLE. |
|
|
Human COR(Cortisol) ELISA Kit |
|
|
Cortisol: emergency hormone, can directly affect immune system and possibly improve the excessive inflammation of autoimmune diseases(e.g. RA and SLE). |
|
|
Human IFN-α(Interferon Alpha) ELISA Kit |
|
|
IFN-α: pleiotropic cytokine, can affect various cell types in lupus. Several genes in interferon pathway are related to lupus risk. The level of IFN-α and expression of IFN responsive gene in lupus patients usually increase. IFN-α may affect lupus clinical features and is the ideal target for lupus treatment. |
|
|
Human IFN-β(Interferon beta) ELISA Kit |
|
|
IFN-β: In SLE, Type I IFN promotes the induction of Type I Interferon-stimulated genes(ISG), and can make B cells produce autoantibodies. Compared with control group, the level of IFN-β in SLE patients' circulating B cell increase. Some patients may benefit from the specific target treatment of IFN-β. |
|
|
Human IFN-γ(Interferon gamma) ELISA Kit |
|
|
IFN-γ: Some mouse model SLE researches show IFN-γ is the key effector of this disease. The cell number of CD4+T producing IFN-γ increases. |
|
REFERENCES
[1]Global epidemiology of systemic lupus erythematosus: a comprehensive systematic analysis and modelling study, PMID: 36241363.
[2]Systemic lupus erythematosus is causally associated with hypothyroidism, but not hyperthyroidism: A Mendelian randomization study, PMID: 36860870.
