Products
SUV39H1 antibody
| Synonyms: | Histone-lysine N-methyltransferase SUV39H1|Histone H3-K9 methyltransferase 1 (H3-K9-HMTase 1)|Lysine N-methyltransferase 1A|Position-effect variegation 3-9 homolog|Suppressor of variegation 3-9 homolog 1 (Su(var)3-9 homolog 1)|SUV39H1|KMT1A|SUV39H antibody | ||
| Catalogue No.: | FNab08404 | Reactivity: | Human, Mouse, Rat |
| Host: | Rabbit | Tested Application: | ELISA, WB, IP |
| Clonality: | polyclonal | Isotype: | IgG |
| Size | Price |
|---|---|
| 100µg | Inquiry |
- SPECIFICATIONS
- FIGURES
- CONDITIONS
- FAQS
- Product Name
- SUV39H1 antibody
- Catalogue No.
- FNab08404
- Size
- 100μg
- Form
- liquid
- Purification
- Immunogen affinity purified
- Purity
- ≥95% as determined by SDS-PAGE
- Clonality
- polyclonal
- Isotype
- IgG
- Storage
- PBS with 0.02% sodium azide and 50% glycerol pH 7.3, -20℃ for 12 months(Avoid repeated freeze / thaw cycles.)
- Immunogen
- suppressor of variegation 3-9 homolog 1(Drosophila)
- Alternative Names
- Histone-lysine N-methyltransferase SUV39H1|Histone H3-K9 methyltransferase 1 (H3-K9-HMTase 1)|Lysine N-methyltransferase 1A|Position-effect variegation 3-9 homolog|Suppressor of variegation 3-9 homolog 1 (Su(var)3-9 homolog 1)|SUV39H1|KMT1A|SUV39H antibody
- UniProt ID
- O43463
- Observed MW
- 48-50 kDa
- Tested Applications
- ELISA, WB, IP
- Recommended dilution
- WB: 1:500-1:2000; IP: 1:200-1:2000
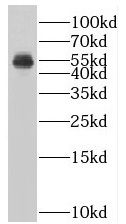 HepG2 cells were subjected to SDS PAGE followed by western blot with FNab08404(SUV39H1 antibody) at dilution of 1:1000
HepG2 cells were subjected to SDS PAGE followed by western blot with FNab08404(SUV39H1 antibody) at dilution of 1:1000
 IP Result of anti-SUV39H1 (IP:FNab08404, 4ug; Detection:FNab08404 1:500) with HeLa cells lysate 2800ug.
IP Result of anti-SUV39H1 (IP:FNab08404, 4ug; Detection:FNab08404 1:500) with HeLa cells lysate 2800ug.
- Background
- Histone methyltransferase that specifically trimethylates 'Lys-9' of histone H3 using monomethylated H3 'Lys-9' as substrate. Also weakly methylates histone H1(in vitro). H3 'Lys-9' trimethylation represents a specific tag for epigenetic transcriptional repression by recruiting HP1(CBX1, CBX3 and/or CBX5) proteins to methylated histones. Mainly functions in heterochromatin regions, thereby playing a central role in the establishment of constitutive heterochromatin at pericentric and telomere regions. H3 'Lys-9' trimethylation is also required to direct DNA methylation at pericentric repeats. SUV39H1 is targeted to histone H3 via its interaction with RB1 and is involved in many processes, such as repression of MYOD1-stimulated differentiation, regulation of the control switch for exiting the cell cycle and entering differentiation, repression by the PML-RARA fusion protein, BMP-induced repression, repression of switch recombination to IgA and regulation of telomere length. Component of the eNoSC(energy-dependent nucleolar silencing) complex, a complex that mediates silencing of rDNA in response to intracellular energy status and acts by recruiting histone-modifying enzymes. The eNoSC complex is able to sense the energy status of cell: upon glucose starvation, elevation of NAD(+)/NADP(+) ratio activates SIRT1, leading to histone H3 deacetylation followed by dimethylation of H3 at 'Lys-9'(H3K9me2) by SUV39H1 and the formation of silent chromatin in the rDNA locus. Recruited by the large PER complex to the E-box elements of the circadian target genes such as PER2 itself or PER1, contributes to the conversion of local chromatin to a heterochromatin-like repressive state through H3 'Lys-9' trimethylation.
How many times can antibodies be recycled?
First, usually it's not suggested to recycle antibodies. After use, buffer system of antibodies has changed. The storage condition of recycled antibodies for different customers also varies. Thus, the performance efficiency of recycled antibodies can’t be guaranteed. Besides, FineTest ever conducted the antibody recycling assay. Assay results show recycling times of different antibodies also varies. Usually, higher antibody titer allows more repeated use. Customers can determine based on experimental requirements.
Notes: After incubation, we recycle rest antibodies to centrifuge tube and store at 4℃. High titer antibodies can be stored for a minimum of one week. Reuse about three times.
What are components of FineTest antibody buffer?
Components of FineTest antibody buffer are usually PBS with proclin300 or sodium azide, BSA, 50% glycerol. Common preservative is proclin300 or sodium azide, which is widely applied in the lab and industry.
How about the storage temperature and duration of FineTest antibodies?
Most antibodies are stored at -20℃. Directly-labeled flow cytometry antibodies should be stored at 2 - 8℃. The shelf life is one year. If after sales issues for purchased antibodies appear, return or replacement is available. Usually, antibodies can be still used after the one-year warranty. We can offer technical support services.
Is dilution required for FineTest antibodies? What’s the dilute solution?
Directly-labeled flow cytometry antibodies are ready-to-use without dilution. Other antibodies are usually concentrated. Follow the dilution ratio suggested in the manual. Dilute solution for different experiments also varies. Common antibody dilution buffers are acceptable(e.g. PBST, TBST, antibody blocking buffer).
How to retrieve antibodies for immunohistochemistry?
Common retrieval buffers: Tris-EDTA Buffer(pH 9.0); Citrate Buffer(pH 6.0)
Heat induced antibody retrieval:
Method 1: Water-bath heating: Put the beaker with retrieval buffer and slide in the boiling water bath. Keep the boiling state for 15min. Naturally cool to room temperature;
Method 2: Microwave retrieval: Put the beaker with retrieval buffer and slide in the microwave oven. Heat at high power for 5min, Switch OFF for 3min, Heat at medium power for 5min. Naturally cool to room temperature.
How to choose secondary antibodies?
(1) Secondary antibodies react with primary antibodies. Thus, secondary antibodies should be against host species of primary antibodies. E.g. If the primary antibody is derived from rabbit, the relevant secondary antibody should be against rabbit. E.g. goat anti rabbit or donkey anti rabbit.
(2) Choose secondary antibody conjugates according to the experimental type, e.g. ELISA, WB, IHC etc. Common enzyme conjugated secondary antibodies are labelled by HRP, AP etc. Fluorescin or dye labelled secondary antibodies are applied in immunofluorescence and flow cytometry(e.g. FITC, Cy3).
