Abstract: Flow cytometry is widely applied in cell phenotype and function. The reliability of experimental results usually depends on the quality of prepared samples, especially high-quality single cell suspension from tissues. The preparation process requires for strictly-controlled enzymolysis conditions to avoid cell injury. Tissue samples(e.g. cancer or immune organs) are important sources for obtaining single cell. High-quality suspension is the basic guarantee for flow cytometry analysis and sorting.
Keywords: Single Cell Suspension, Flow Cytometry, Mechanical Dissection, Enzymolysis
1. Preparation Method
Single cell suspension preparation uses mechanical dissection and digestive enzymes. Mechanical dissection physically dissociates tissues via splicing, grinding and filtration, suitable for soft tissues like spleen and lymph nodes. The operation is easy and rapid. Samples with dense structures or abundant connective tissues require for digestive enzymes to improve dissociative effects. Mechanical dissection has higher efficiency but easily damages cell or incompletely dissociates.
2. Tissue Preparation
Tissue preparation should choose fresh samples and avoid cryopreservation to reduce cell loss. Shred tissues with scissors or scalpels to help enzymatic digestion via increasing surface area. Common enzymes include Collagenase, Dispase, Hyaluronidase etc. TrypLE is better than Trypsin via reserving membrane antigen and can help DNase I and EDTA prevent cell aggregation. Accutase is the complex enzyme featured with high cell recovery and reserved antigen.
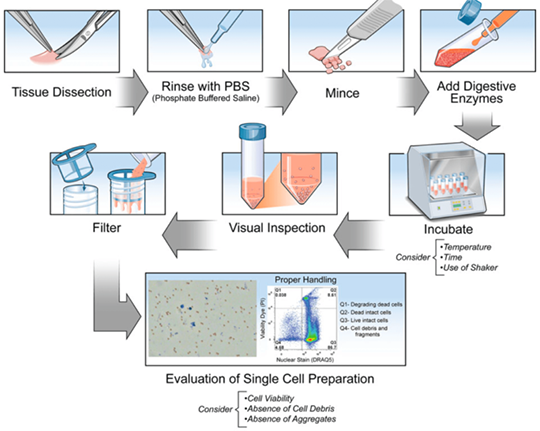
3. Precise Control of Time and Temperature
During enzymolysis, time and temperature should be property controlled. The activity of most enzymes at 37℃ is the best. But low temperature(e.g. 4℃) can decrease cell death. The incubation duration should be properly extended. The digestion duration should be moderate to avoid shorter duration-induced cell aggregation or longer duration-induced cell damage. Mechanical devices(e.g. orbital shaker) can accelerate tissue dissociation. Finally, remove undigested tissue block to obtain high-quality single cell suspension with 100 μm filter.
4. Evaluation of Single Cell Suspension
Cell activity detection usually uses trypan blue staining and counts dead cells under microscope. The survival rate should be higher than 80%. Flow cytometry can recognize debris via nucleic acid dye, and accurately evaluate ratio of dead cells via viability dye(e.g. propidium iodine). In order to remove dead cells, debris and aggregates, Miltenyi or STEMCELL magnetic bead sorting kit can be used. Lysing red blood cell in the sample can ensure the experimental consistency.
5. Notes
5.1. Centrifugal Parameters and Operation
Use RCF instead of RPM. 300-400 RCF is recommended for living cells. Cell fixation can achieve 900 RCF. Higher RCP can damage cells. Choose polypropylene centrifuge tubes to avoid vortex operation. Gently pipetting to improve survival rate.
5.2. Special Experimental Requests
Phosphorylated flow cytometry requires addition of phosphatase inhibitors to protect proteins. During RNA detection, prevent RNA degradation with RNase inhibitors.
5.3. Cryopreservation and Fixation
The survival rate of frozen cells is low and decreases to 33.6%, just 54% of non-frozen cells. 4% PFA can stabilize cells but may change antigen conformation. Pilot assay is required for validation.
Since digestion among tissues and species is different, optimization of preparing single cell suspension depends on tissue type, selected enzyme, digestion time and temperature. These variables can directly affect experimental quality. Thus, it's suggested to regulate according to citations and experimental experiences.
| Recommended Products | |||
| Species | Cell Populations | Flow Cytometry Antibody Combination | Cat.No |
| Human | T/B/NK cell populations detection | CD45-PerCP | PCP-30039 |
| CD3-FITC | FITC-30004 | ||
| CD16-PE | PE-30061 | ||
| CD56-PE | PE-30008 | ||
| CD19-APC | APC-30066 | ||
| Human | Thl/Th2 cell populations detection | CD3-PerCP/Cyanine5.5 | PCP55-30004 |
| CD4-FITC | FITC-30005 | ||
| IFN-γ-PE | PE-30053 | ||
| IL4-APC | APC-30043 | ||
| Mouse | Thl/Th2 cell populations detection | CD3-PerCP/Cyanine5.5 | PCP55-30002 |
| CD4-FITC | FITC-30128 | ||
| IFN-γ-PE | PE-30074 | ||
| IL4-APC | APC-30026 | ||
| Human | Treg cell populations detection | CD4-FITC | FITC-30005 |
| CD25-PE | PE-30035 | ||
| CD3-PerCP-Cy5.5 | PCP55-30004 | ||
| CD127-FineTest®647 | F647-30033 | ||
| Mouse | Treg cell populations detection | CD4-FITC | FITC-30128 |
| CD25-APC | APC-30017 | ||
| FOXP3-PE | PE-30111 | ||
REFERENCES
[1]Microdissection and Single-Cell Suspension of Neocortical Layers From Ferret Brain for Single-Cell Assays, PMID: 39735300.
[2]Mouse Tail-Skin Dissociation and Preparation of Live Single-Cell Suspension for Downstream Analysis of Melanocytes, PMID: 39625901.
