Abstract: Panel design plays an important role in flow cytometry and determines the quality of experimental results. The accuracy of experimental results is affected by cell surface antigen, antibody selection, fluorochrome labelling and reagent combination. With the technical development, modern flow cytometers can synchronously detect over 40 parameters and analyse more complex problems. Proper panel design for valuable samples can obtain more sufficient data to promote further research.
Keywords: Multicolor Panel Design, Flow Cytometry, Flow Cytometers, Fluorescence Labeling, Fluorescent Marker, Colour Matching
1. Investigation of Detected Markers
Investigation of detected markers is important for any panel design. Before the experiment, understanding markers related to the cell can determine the labelling and detection method. Markers for the same cell in different species(e.g. human, mouse) may be varied. It's suggested to check related citations. Besides, OMIPs, BioLegend official website and CD molecule are also references for panel design, ensuring accuracy and reproducibility of the experiment.
2. Types of Antigen Expression Level
Antigen expression includes low, medium and high level. Besides, three types based on the expression pattern are shown in the table below.

3. Configuration of Flow Cytometers
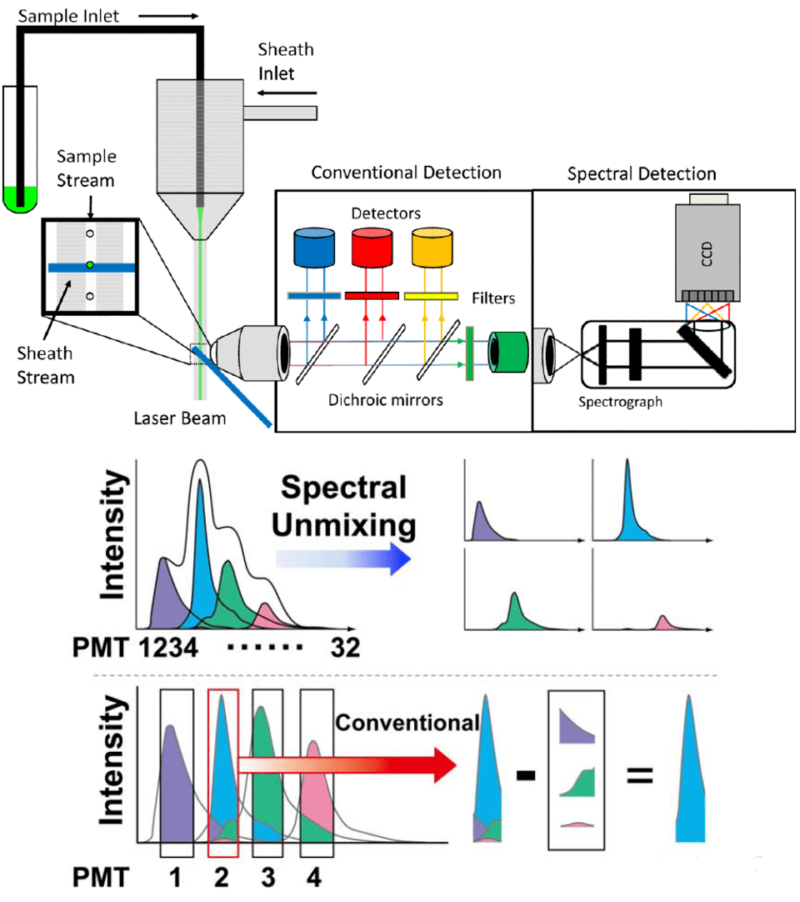
Current flow cytometers include traditional, spectral and mass cytometers. These devices follow similar panel design, but data reading is different. Traditional cytometers receive fluorescence signal via dichroic mirrors and optical filter. Emission spectrum of fluoresceins is usually crossed, leading to fluorescent leak. Thus, multicolor assay needs to calculate fluorescent leak ratio with single staining tubes.
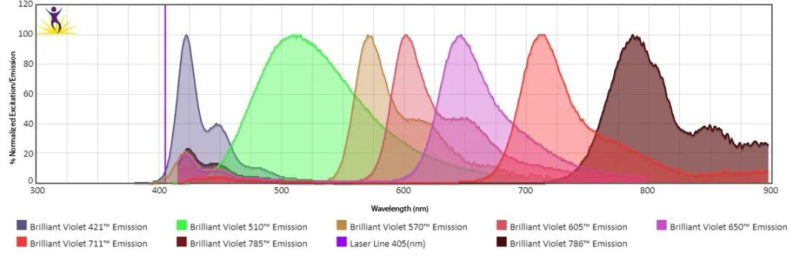
Spectral cytometers can collect full spectrum characteristics of fluoresceins. Each fluorescein has its unique spectral property, similar to fingerprint. Spectral cytometers can efficiently distinguish different fluoresceins via spectral analysis and improve accuracy of multicolor assay.
Mass cytometers label cells via stable isotopes, and detect ion mass ratio via TOF-MS.
4. Selection of Fluoresceins
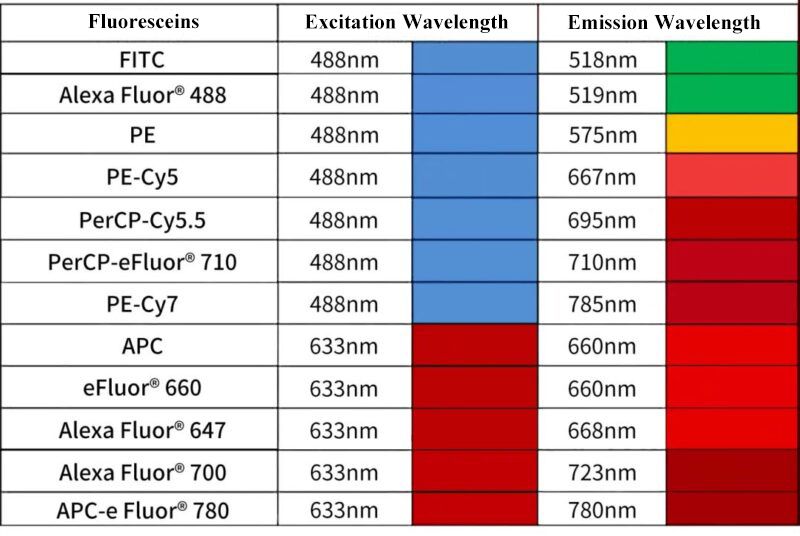
When selecting fluorescent antibodies based on device configuration, fluorescent brightness can be judged via staining index. Common fluoresceins are shown in the table below, but more fluoresceins are practically available. The development of new dyes provides more choices(e.g. BD RealBlue and RealYellow fluoresceins).
5. Colour Matching for Antigen and Fluoresceins
Colour matching should follow several basic principles. First, strength and weakness combination: assign highly expressed markers to low brightness fluoresceins. Assign markers with low or unknown expression level to high brightness fluoresceins. Besides, characteristics of fluoresceins should be considered. Easily quenched fluoresceins are unsuitable for fixation and permeabilization of markers. Dye pairs with low spillover are applicable for co-expressed markers and can avoid error diffusion. Mutually exclusive expression markers choose strong interference fluoresceins. For great fluorescein leak, assign to markers lowly expressed in specific cells. Tissue samples use viability dyes.
6. Testing of Multicolor Panel Design
Before testing, it's noted that: First, antibody titration is very important. Concentration recommended by the manufacturer is just for reference. The best concentration depends on titration. Second, suitable control is more important, especially for compensating single staining and FMO control.
If you thought the colour matching was very complex, please ask for technical support from our company. FineTest provides free colour matching, assay guidance and data analysis services etc, committed to improving efficiency and accuracy of scientific research.
7. Recommended Products
| Species | Cell Populations | Flow Cytometry Antibody Combination | Cat.No |
| Human | T/B/NK cell populations detection | CD45-PerCP | PCP-30039 |
| CD3-FITC | FITC-30004 | ||
| CD16-PE | PE-30061 | ||
| CD56-PE | PE-30008 | ||
| CD19-APC | APC-30066 | ||
| Human | Thl/Th2 cell populations detection | CD3-PerCP/Cyanine5.5 | PCP55-30004 |
| CD4-FITC | FITC-30005 | ||
| IFN-γ-PE | PE-30053 | ||
| IL4-APC | APC-30043 | ||
| Mouse | Thl/Th2 cell populations detection | CD3-PerCP/Cyanine5.5 | PCP55-30002 |
| CD4-FITC | FITC-30001 | ||
| IFN-γ-PE | PE-30074 | ||
| IL4-APC | APC-30026 | ||
| Human | Treg cell populations detection | CD4-FITC | FITC-30005 |
| CD25-PE | PE-30035 | ||
| CD127-APC | APC-30033 | ||
| Mouse | Treg cell populations detection | CD4-FITC | FITC-30001 |
| CD25-PE | PE-30017 | ||
| FOXP3-APC | APC-30055 |
REFERENCES
[1]OMIP-112: 42-Parameter (40-Color) Spectral Flow Cytometry Panel for Comprehensive Immunophenotyping of Human Peripheral Blood Leukocytes, PMID: 40095400.
[2]Deep phenotyping of human neutrophils in whole blood using a 33-color spectral flow cytometry panel, PMID: 40244916.
