Abstract: Intracellular staining and molecular detection can help identification of cell subpopulation and status. Unlike traditional staining of antibodies on the surface, flow cytometry analysis of intracellular proteins requires for fixation and permeabilization of cells to achieve antibody-antigen binding. Flow cytometry mass spectrometry can detect cell surface markers and analyzes components in cells and nucleus. Multi-channel can synchronously detect multiple parameters without voltage regulation and compensation, greatly improving efficiency.
Keywords: Flow Cytometry Protocol, Intracellular Staining, Nuclear Factor Staining
1. Intracellular Staining Solution
Steps of intracellular cytokine staining are specified below:
1.1. Cell Activation and Stimulation
The expression of T and B cytokines in resting conditions is very low. In vitro activation induces cytokine production. Common stimulators are PMA and Ionomycin, promoting activation of T cell via activated PKC. Addition of protein transport inhibitors(e.g. Brefeldin A or monensin) during stimulation prevents secretion of cytokines and keeps intracellular accumulation. Stimulation lasts for 4-6h at 37℃ and 5% CO2.
1.2. Cell Fixation
After staining of cell surface markers, add fixation buffer to fix cells. Incubate for 20min at room temperature in the dark. Centrifuge to remove the supernatant. Cells can be temporarily stored at 4℃ or -80℃(Proper preservation solution is required.).
1.3. Cell Permeabilization
Resuspend and fix cells with 1× permeabilization wash buffer. Centrifuge for 5-10min at 350×g. Discard supernatant and wash again. Antibodies can enter cells and bind with the target antigen after permeabilization.
1.4. Intracellular Staining
Resuspend cells with 1× permeabilization wash buffer. Add relevant antibodies and incubate for 20min at room temperature in the dark. Using biotinylated antibody should add FITC-conjugated streptavidin for incubation. Then, wash twice and discard supernatant.
1.5. Sample Preparation and Detection
Resuspend cells with cell staining buffer. Set control and perform flow cytometry analysis. Add unlabeled antibodies or recombinant cytokines to block and remove non-specific binding.
2. Nuclear Factor Staining
Experimental Results of FineTest K081 Kit:
1) Add 100 μL cell/tube(about 1×106 cells) into flow cytometry tubes.
2) [Optional Step] Perform staining with Fixable Viability Dye according to manual.
3) [Optional Step] Block cell suspension with Fc receptor blocker according to experimental requirements.
4) Stain cell surface markers with fluorescent labeled antibodies according to antibody manual.
5) After incubation, add 1mL cell staining buffer[K079]. Centrifuge for 5min at 300×g and discard supernatant. Then, add 100uL cell staining buffer[K079] to resuspend cells.
6) Add 1mL 1× fixation working solution and mix well via vortex. React for 30min at 4℃. Centrifuge for 5min at 600×g and discard supernatant.
7) Add 2mL 1× permeabilization working solution and mix well via vortex. Centrifuge for 5min at 600×g and discard supernatant.
8) Repeat step 7.
9) Add 100μL 1× permeabilization working solution to resuspend cells.
10) Stain nucleus with fluorescent labeled antibodies according to antibody manual. Mix well and incubate for 30min at room temperature in the dark.
11) Add 2mL 1× permeabilization working solution. Centrifuge for 5min at 600×g and discard supernatant.
12) Add proper amount of cell staining buffer[K079] to resuspend cells for further detection.
3. Classical Case
Staining case for foxp3 in mouse spleen.(Detection result for FineTest K081 Kit):
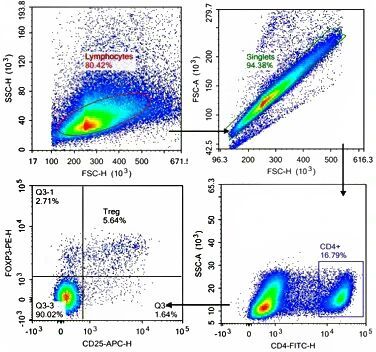
Detection of CD25 in CD4⁺ T cell population and high expression of FOXP3 can recognize regulatory T cell(Tregs). Staining for surface markers and intracellular transcription factors effectively distinguish T cell population with strong immune regulation.
4. Conclusion
Staining and detection of intracellular molecules can significantly recognize cell subpopulation and analyze the function. Unlike traditional staining for cell surface antigens, intracellular staining should fix cells first to stabilize structure and cross-link proteins. Permeabilization allows the entry of antibodies into cells for further binding with intracellular or nuclear antigen.
| Recommended Products | |||
| Species | Cell Populations | Flow Cytometry Antibody Combination | Cat.No |
| Human | T/B/NK cell populations detection | CD45-PerCP | PCP-30039 |
| CD3-FITC | FITC-30004 | ||
| CD16-PE | PE-30061 | ||
| CD56-PE | PE-30008 | ||
| CD19-APC | APC-30066 | ||
| Human | Thl/Th2 cell populations detection | CD3-PerCP/Cyanine5.5 | PCP55-30004 |
| CD4-FITC | FITC-30005 | ||
| IFN-γ-PE | PE-30053 | ||
| IL4-APC | APC-30043 | ||
| Mouse | Thl/Th2 cell populations detection | CD3-PerCP/Cyanine5.5 | PCP55-30002 |
| CD4-FITC | FITC-30128 | ||
| IFN-γ-PE | PE-30074 | ||
| IL4-APC | APC-30026 | ||
| Human | Treg cell populations detection | CD4-FITC | FITC-30005 |
| CD25-PE | PE-30035 | ||
| CD3-PerCP-Cy5.5 | PCP55-30004 | ||
| CD127-FineTest®647 | F647-30033 | ||
| Mouse | Treg cell populations detection | CD4-FITC | FITC-30128 |
| CD25-APC | APC-30017 | ||
| FOXP3-PE | PE-30111 | ||
REFERENCES
[1]In Vitro Culture for H5N1-Specific Duck T Cells and Detection of Immune Responses Using Intracellular Cytokine Staining Method, PMID: 40522882.
[2]Optimized protocol for intracellular labeling of red blood cells with anti-hemoglobin F for confocal microscopy analysis, PMID: 40939419.
