Abstract: Flow cytometry is widely applied in immunology and hematology. Difficulties for analyzing brain tissue include complex cell interaction, high-fat induced interference with myelin debris and autofluorescence in brain tissue. Deep investigations on optimizing flow cytometry analysis for brain tissue have been conducted for improving accuracy and reliability, including cell separation, labeling method and data correction.
Keywords: Flow Cytometry Analysis, Brain Tissue, Removal of Myelin Debris, Autofluorescence, Enzymatic Effects
1. High-efficient Removal of Myelin Debris
The research optimizes flow cytometry analysis for mouse brain tissue: take off cortex, hippocampus and corpus callosum; digest with collagenase or papain via mechanical shearing. Gradient centrifugation via different concentrations of isosmotic solution(e.g. Percoll) shows 24% SIP can effectively remove myelin debris at 400g, 25min and 18℃. Cell survival rate is 70% and residual is minimum. Erythrocyte lysis after centrifugation produces high-quality cell suspension suitable for flow cytometry.
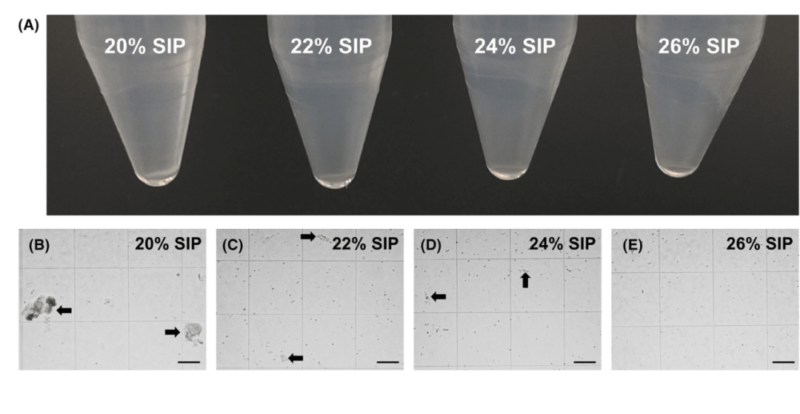
2. Autofluorescence in Different Regions
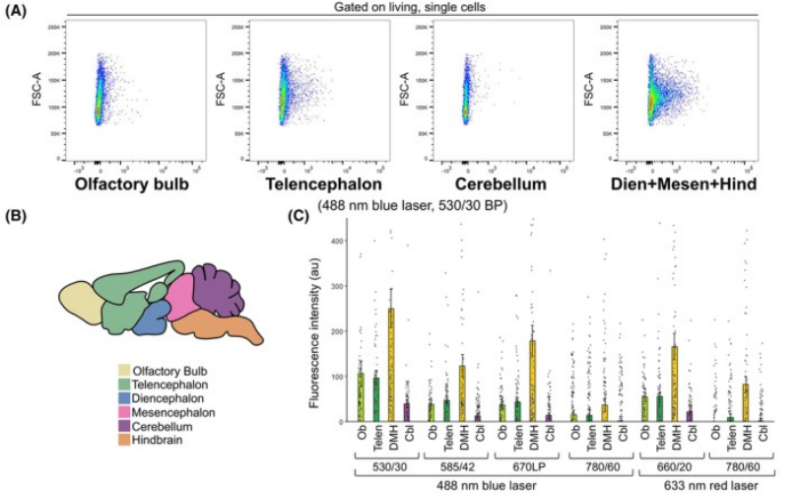
In flow cytometry analysis, autofluorescence is the key factor affecting detection accuracy of brain tissue. Lipofuscin is the main source. This research obtains unlabeled sample from eight-day postnatal mouse brain, including four regions olfactory bulb, telencephalon, cerebellum, DMH(diencephalon+midbrain+hindbrain). The result shows the strongest autofluorescence of DMH region under multiple lasers and optical filters(Figure A and C), indicating the obvious background interference. Blue laser at 488 nm and 530/30 BP optical filters can also show higher autofluorescence in olfactory bulb and telencephalon(Figure C). Thus, relevant fluorescence compensation and background correction for different regions are required for improving detection sensitivity and data reliability during analysis of brain tissue.
3. Enzymatic Effects on Cell Viability
Comparisons among effects of collagenase, papain and accutase on brain cells show over 80% survival rate of microglia is not affected by enzymatic types. Survival rate of neuron and oligodendrocyte processed by papain is obviously higher than collagenase, showing the gentle enzymatic characteristics. Apoptotic ratio of astrocyte is always higher no matter which enzyme is used. More gently separation method is required.
4. Selection and Validation of Neuronal Markers
Specificity of various antibodies is validated with screening of flow cytometry and GFP expression driven by AAV carrier and neuron specific promoters(e.g. NSE, GAD65). During surface labeling, NCAM requires for permeabilization but signal is stable(+++). The specificity of CD200 is medium(+) without permeabilization. Intracellular labeling for NeuN should optimize permeabilization conditions. Specificity is higher(++). Neuron specificity of GABA labeled by GAD65 is strong(+) but conditions are strict. Validation of AAV-GFP confirms antibodies targeting CD200, NCAM or NeuN can efficiently recognize neuron. MAP2ab is removed due to overlapped signal.
Flow cytometry analysis for brain tissue should focus on three key points: Gradient centrifugation with 24% SIP, compatible for cell yield and purity. Correct autofluorescence in different brain regions and reduce background interference; Select markers(e.g. CD200, NCAM or NeuN) according to target cells and optimize permeabilization conditions. In the future, better fixation method and separation protocol for gentle astrocytes can improve analytical accuracy further.
| Recommended Products | |||
| Species | Cell Populations | Flow Cytometry Antibody Combination | Cat.No |
| Human | T/B/NK cell populations detection | CD45-PerCP | PCP-30039 |
| CD3-FITC | FITC-30004 | ||
| CD16-PE | PE-30061 | ||
| CD56-PE | PE-30008 | ||
| CD19-APC | APC-30066 | ||
| Human | Thl/Th2 cell populations detection | CD3-PerCP/Cyanine5.5 | PCP55-30004 |
| CD4-FITC | FITC-30005 | ||
| IFN-γ-PE | PE-30053 | ||
| IL4-APC | APC-30043 | ||
| Mouse | Thl/Th2 cell populations detection | CD3-PerCP/Cyanine5.5 | PCP55-30002 |
| CD4-FITC | FITC-30128 | ||
| IFN-γ-PE | PE-30074 | ||
| IL4-APC | APC-30026 | ||
| Human | Treg cell populations detection | CD4-FITC | FITC-30005 |
| CD25-PE | PE-30035 | ||
| CD3-PerCP-Cy5.5 | PCP55-30004 | ||
| CD127-FineTest®647 | F647-30033 | ||
| Mouse | Treg cell populations detection | CD4-FITC | FITC-30128 |
| CD25-APC | APC-30017 | ||
| FOXP3-PE | PE-30111 | ||
REFERENCES
[1]Flow Cytometry and Single-Cell Analysis for Characterizing Microglia Activation in Early Postnatal Mouse Brain Development, PMID: 41115126.
[2]Flow Cytometry Identification of Cell Compartments in the Murine Brain, PMID: 37355546.
