Abstract: Controls play an important role in flow cytometry. Accurate and reliable results can’t be obtained without proper controls. First, signals are relative(e.g. diffused light and fluorescence signal). Thus, blank or unstained control is required to define signal range. Second, during setting gating of the target cell population, various controls help to determine the gating position. Finally, controls can evaluate effectiveness of experimental operation. Commons FACS controls include blank control, unstained control, positive control and negative control.
Keywords: FACS Control, Flow Cytometry, Blank Control, Compensation Control, Isotype Control, Controls in Flow Cytometry
1. Blank Control
Background fluorescence or auto-fluorescence in flow cytometry usually distinguishes cell type, and helps to regulate voltage or gain of the device, thereby confirming the probable position of negative cell population. However, these controls in multicolour assay are usually not applied in accurate distinction between negative and positive cell population. Researchers usually accurately distinguish different cell populations via more accurate negative and positive control. Appropriate controls are very important to get reliable experimental results.
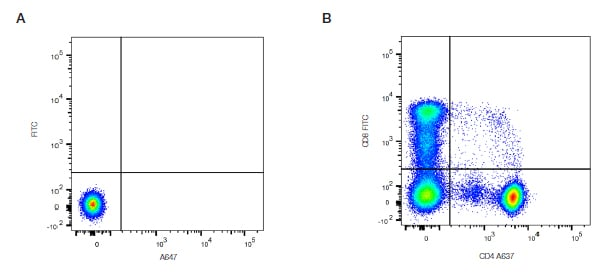
2. Compensation Control
Compensation control mainly regulates fluorescence compensation. The number of fluorescence dyes used in fully-stained samples determines the number of single-stained tubes. Compensation control can use cells or microspheres, e.g. For double-stained samples with FITC and PE, single-stained tubes for FITC, PE and unstained-control tube are required to ensure accurate compensation setting, effectively reducing overlapped fluorescence signal-induced interference to improve the accuracy of experimental results.
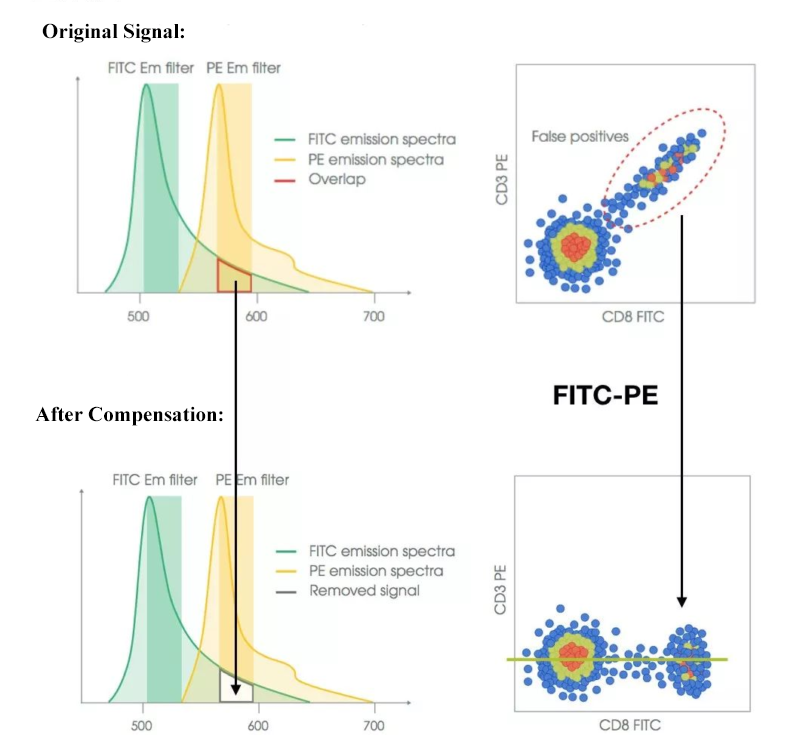
3. FMO (Fluorescence Minus One) and Biological Control
Setting proper control group plays a key role in successful flow cytometry, and can effectively avoid false positive and negative results. Five types of controls should be included in flow cytometry at least: blank control, isotype control, single-stain control, FMO (fluorescence minus one) control, biological control. These controls contribute to instrument calibration, evaluation of background signal, confirmation of compensation value, validation of rational experimental design to improve data accuracy and reliability. The meticulous setting of control groups can ensure the validity and reproducibility of experimental results.
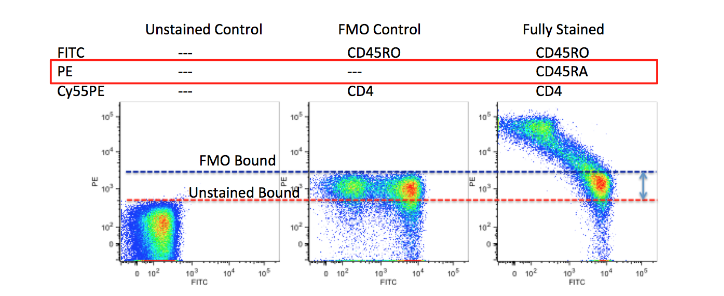
4. Isotype Control
Isotype control refers to antibodies without specific binding with the target(irrelevant to Fab fragment), sharing the same species, subtype and fluorescent marker as flow cytometry antibodies. In cells expressing Fc receptor on the surface(e.g. monocyte, dendritic cell, macrophage etc), non-specific binding with Fc fragment and some fluorescence dyes may happen. Thus, isotype control usually removes non-specific staining. However, setting of gating range in multicolour assay usually uses FMO control instead of Isotype control.
5. How to Select Controls in Flow Cytometry
In flow cytometry, blank and single positive control can regulate voltage and compensation. Other controls assist to distinguish negative and positive cell population. Control type depends on experimental type, e.g. apoptosis assay usually requires for blank control, single-stain control and biological control.
6. FCM Colour Matching for Immunocyte Subpopulations
| Recommended Flow Cytometry Antibodies | |||
| Species | Cell Populations | Flow Cytometry Antibody Combination | Cat.No |
| Human | T/B/NK cell populations detection | CD45-PerCP | PCP-30039 |
| CD3-FITC | FITC-30004 | ||
| CD16-PE | PE-30061 | ||
| CD56-PE | PE-30008 | ||
| CD19-APC | APC-30066 | ||
| Human | Thl/Th2 cell populations detection | CD3-PerCP/Cyanine5.5 | PCP55-30004 |
| CD4-FITC | FITC-30005 | ||
| IFN-γ-PE | PE-30053 | ||
| IL4-APC | APC-30043 | ||
| Mouse | Thl/Th2 cell populations detection | CD3-PerCP/Cyanine5.5 | PCP55-30002 |
| CD4-FITC | FITC-30001 | ||
| IFN-γ-PE | PE-30074 | ||
| IL4-APC | APC-30026 | ||
| Human | Treg cell populations detection | CD4-FITC | FITC-30005 |
| CD25-PE | PE-30035 | ||
| CD127-APC | APC-30033 | ||
| Mouse | Treg cell populations detection | CD4-FITC | FITC-30001 |
| CD25-PE | PE-30017 | ||
| FOXP3-APC | APC-30055 | ||
REFERENCES
[1]Flow cytometry identifies changes in peripheral and intrathecal lymphocyte patterns in CNS autoimmune disorders and primary CNS malignancies, PMID: 39497174.
[2]Nanoscale flow cytometry-based quantification of blood-based extracellular vesicle biomarkers distinguishes MCI and Alzheimer's disease, PMID: 38958575.
