Abstract: Atopic dermatitis(AD) is the chronic relapsing inflammatory dermatosis with severe pruritus and recurrent rash, greatly affecting patients’ quality of life. This disease coexists with some atopic disorders(e.g. hypersensitive rhinitis, asthma etc), showing abnormal features of immune system. Urgent treatment demands for moderate and severe patients are relief of persistent pruritus, rapid control of skin inflammation and decreased recurrence. In recent years, targeted therapy for AD is gradually varied. Advancements in antibody therapy are obvious.
Keywords: Atopic Dermatitis, Chronic Inflammation, Targeted Therapy, Skin Inflammation, Immune System Disorders
1. Clinical Atopic Dermatitis Symptoms
Clinical symptoms of atopic dermatitis are various, including dry skin, recurrent eczematoid skin lesions and severe pruritus. Skin lesions are often found in wrinkled areas(e.g. antecubital fossa, popliteal fossa, neck etc), face, four limbs, body and hands. Most skin lesions are subacute or chronic dermatitis accompanied with dryness and hypertrophy. Some patients may suffer from prurigo-like changes and protracted disease.
2. Pathogenesis of Atopic Dermatitis
Atopic dermatitis(AD) is involved in many factors, e.g. heredity, immunity, infection and environment etc. Damage of skin barrier and immune imbalance are key mechanism. AD patients have multiple dysfunctions in physics, microorganism, chemistry and immunologic barrier. Lipid decrease of epidermal structural proteins(e.g. filaggrin, loricrin) and ceramide makes skin barrier weaker. Stimulated by environmental allergen or pathogen, keratinocyte can release pro-inflammatory factors(e.g. TSLP, IL-25, IL-33, TNF-α etc). Activation of dendritic cells and type 2 innate lymphoid cells can cause IL-4 and IL-13 dominated inflammatory response. The inflammation inhibits structural protein synthesis further, leading to a vicious circle for persistent and recurrent AD inflammation.
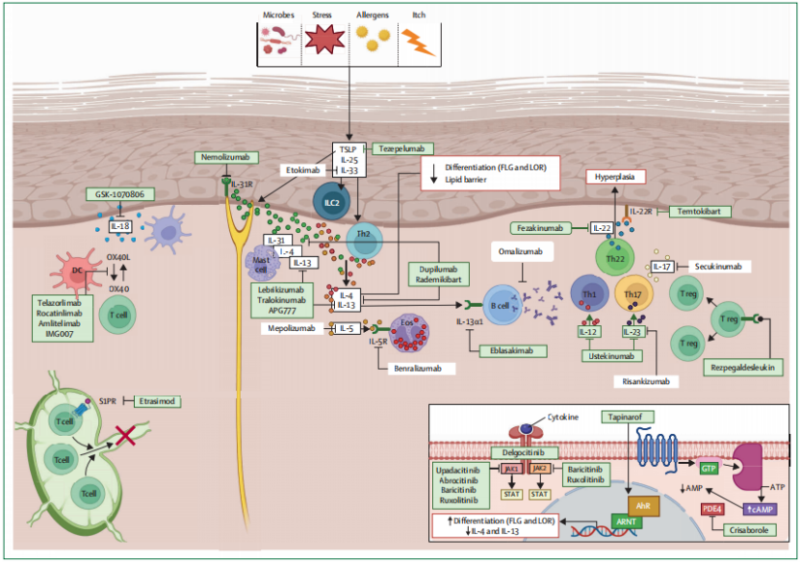
3. Targeted Therapy Drugs
With the deeper research on AD mechanism, Th2 inflammatory factors targeting IL-4/IL-13, downstream JAK-STAT pathway inhibitors and small molecule drugs have been hot research topics.
3.1. IL-4Rα Targeted Drugs
The increase of IL-4 and IL-13 in AD patients’ peripheral blood and skin lesions is obvious, and closely related to severity of the disease. Dupilumab is the first and unique biological reagent targeting IL-4Rα. Blocking the binding among IL-4, IL-13 and their receptors can effectively inhibit type 2 inflammation.
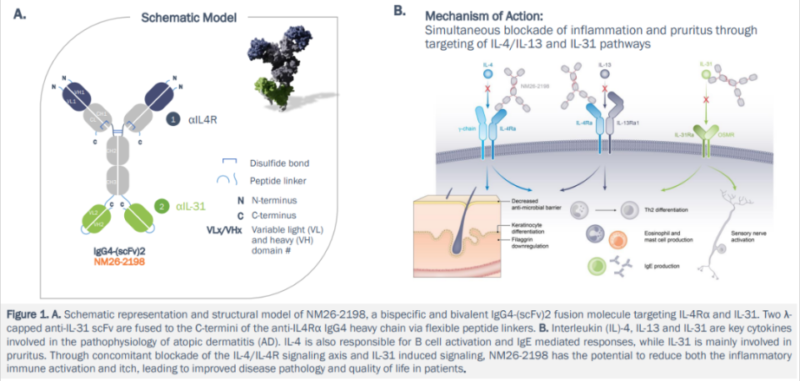
3.2. NM26 Targeting IL-4Rα/IL-31
NM26(NM26-2198) is the bispecific antibody targeting IL-4Rα and IL-31. Blocking Th2 inflammation and neuropathic pruritus can improve inflammatory response and skin barrier function. This drug was acquired by Johnson & Johnson and is now in clinical trial phase II. In the future, it may be the new AD therapy with higher efficiency.
3.3. Biological Reagents for Other Targets
Th2 related factors(e.g. IL-22, IL-33, OX40 etc) play an important role in the development of AD. Some targeted drugs have been in the R&D process. Fezakinumab is the human IgG1 monoclonal antibody, and can bind with IL-22 to prevent the binding with receptor. Clinical results in Ⅱa phase show this drug can obviously improve patients’ SCORAD mark after 20 weeks of treatment. The curative effect is better than placebo group.
4. JAK Inhibitors
Roles of JAK-STAT pathway in AD immune dysfunction are very important, including overreaction of Th2, activation of eosinophils, maturation of B cell, functional inhibition of Tregs etc. Activated JAK can promote the entry of phosphorylated STAT into nucleus, aggravating the disease via expression of inflammatory gene. Currently, five kinds of JAK inhibitors around the world have been approved for AD treatment. JAK1 small molecule drugs approved by China are upadacitinib and abrocitinib. Curative effects in moderate and severe AD are better.
Atopic dermatitis is the complex disease and facing rapid change. Deeper understanding of pathogenesis and immune regulation promotes the development of new drugs into advanced clinical stage. In the future, patients’ diseases can be safely controlled via targeted therapy.
5. Recommended Products
| Recommended Antibodies | |
| Cat.No | Product Name |
| FNab04279 | IL4 antibody |
| FNab10102 | IL4R antibody |
| FNab10489 | IL13 antibody |
| FNab10882 | IL22 antibody |
| FNab04269 | IL31 antibody |
| FNab09047 | TSLP antibody |
| Recommended Recombinant Proteins | |
| Cat.No | Product Name |
| P4505 | Recombinant Human IL-4 |
| Pr22572 | Recombinant Human IL-4RA |
| Pr22190 | Recombinant Human IL-13 |
| P6627 | Recombinant Human IL-22 |
| P6017 | Recombinant Human IL-31 |
| P6348 | Recombinant Human TSLP |
| Pr10142 | Recombinant Mouse IL-4 |
| Pr22529 | Recombinant Mouse IL-4RA |
| P4879 | Recombinant Mouse IL-13 |
| P6731 | Recombinant Mouse IL-22 |
| P6016 | Recombinant Mouse IL-31 |
| Pr23162 | Recombinant Mouse TSLP |
| P5908 | Recombinant Rat IL-22 |
| Recommended ELISA Kits | |
| Cat.No | Product Name |
| EH0199 | Human IL-4 ELISA Kit |
| EH3266 | Human IL-13 ELISA Kit |
| EH0191 | Human IL-22 ELISA Kit |
| QT-EH0191 | Human IL-22 QuickTest ELISA Kit |
| EH0197 | Human IL-31 ELISA Kit |
| QT-EH0197 | Human IL-31 QuickTest ELISA Kit |
| EH0322 | Human TSLP ELISA Kit |
| EM0119 | Mouse IL-4 ELISA Kit |
| QT-EM0119 | Mouse IL-4 QuickTest ELISA Kit |
| EM0103 | Mouse IL-13 ELISA Kit |
| QT-EM0103 | Mouse IL-13 QuickTest ELISA Kit |
| EM1810 | Mouse IL-22 ELISA Kit |
| EM1900 | Mouse IL-31 ELISA Kit |
| EM0201 | Mouse TSLP ELISA Kit |
| QT-EM1388 | Mouse TARC QuickTest ELISA Kit |
| ER0041 | Rat IL-4 ELISA Kit |
| QT-ER0041 | Rat IL-4 QuickTest ELISA Kit |
| QT-ER0034 | Rat IL-13 QuickTest ELISA Kit |
| ER1630 | Rat IL-22 ELISA Kit |
| EMK0269 | Monkey IL-31 ELISA Kit |
| QT-ER1412 | Rat TSLP QuickTest ELISA Kit |
| QT-ER1364 | Rat TARC QuickTest ELISA Kit |
REFERENCES
[1]Comparative efficacy and safety of ilunocitinib and oclacitinib for the control of pruritus and associated skin lesions in dogs with atopic dermatitis, PMID: 39757965.
[2]IL-24 promotes atopic dermatitis-like inflammation through driving MRSA-induced allergic responses, PMID: 38752989.
