Abstract: Overnight antibody staining is suitable for detecting weakly expressed antigen or low affinity antibody. Extended incubation duration can improve antigen-antibody binding, signal resolution, sensitivity and data accuracy. Besides, overnight staining can help to decrease background interference, experimental variability and antibody volume. Overnight staining can obviously improve detection effects of complex sample, stability and reproducibility.
Keywords: Overnight Antibody Staining, Flow Cytometry, Incubation, Antigen-antibody Binding
1. Surface and Intracellular Staining Protocol
Surface and intracellular staining steps are different and specified below.
Cell Preparation: 2 million cells are used in each sample. FACS buffer is PBS + 2.5% FCS + 2 mM EDTA.
Blocking: Block mouse derived cells with CD16/32 antibody for 30min. Block human derived cells with Fc.
Surface Staining: Add fluorescent antibody. Incubate at 4℃ for 16h (30-60min control).
Intracellular staining: Stimulate cells. Finish Surface Staining → Fixation → Permeabilization → Incubate overnight via adding antibodies into permeabilization buffer → Wash and test in the next day
2. Improvement for Experimental Effects
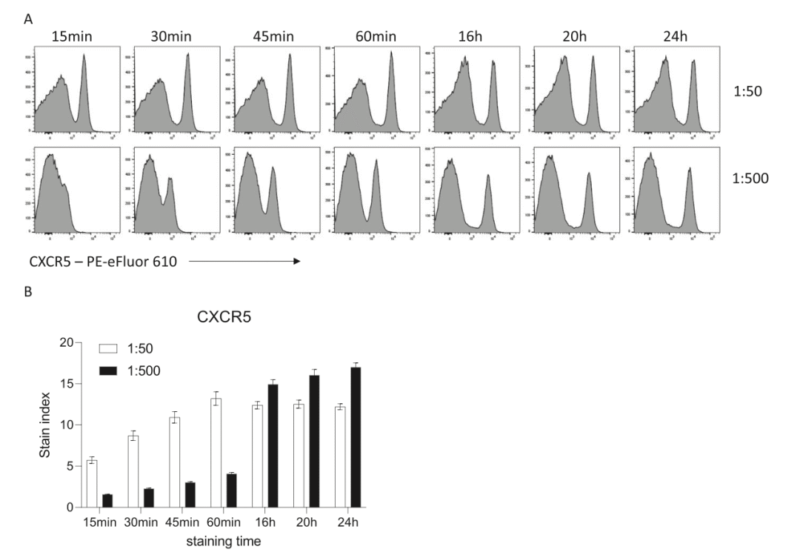
Researches show extended staining duration can obviously improve fluorescent signal. Take surface staining of mouse splenocyte with CXCR5 for example. Compare staining effects among 15min, 30min, 60min and 16h. Fluorescence intensity increases with incubation duration. Staining for 16h can reduce one-tenth of antibody volume. The signal intensity is equivalent to staining for 30min. Extended duration can help to improve sensitivity and decrease antibody volume.
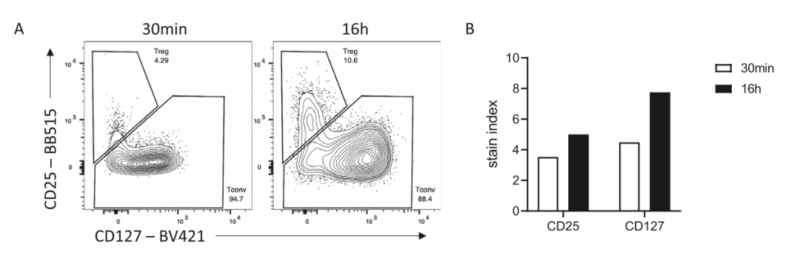
In detecting human regulatory T cell, signal intensity of overnight staining for CD25 and CD127 is stronger than 30min staining. Gating of memory T cell population is clearer.
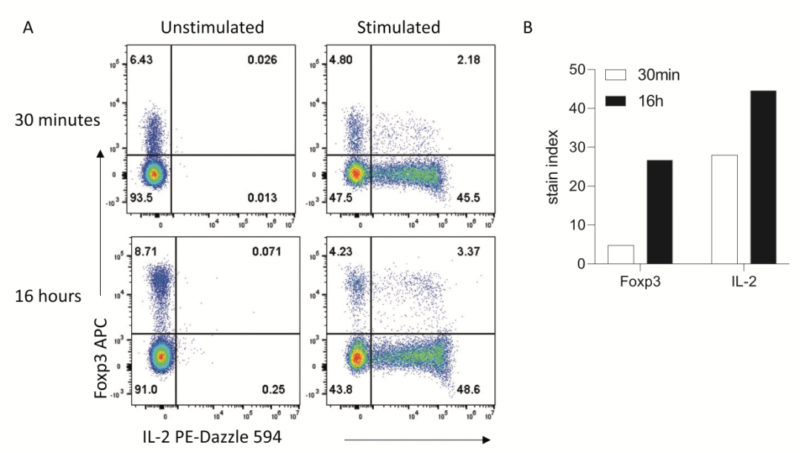
3. Intracellular Staining
Overnight staining is suitable for surface antigen, and can also improve detection sensitivity for intracellular or even nuclear proteins(e.g. IL-2 and Foxp3). E.g. in the stimulation experiment of permeated mouse splenocyte, the signal intensity of stained IL-2 overnight is stronger than 30min staining. Non-specific signal is not found in unstimulated control group. Thus, this method improves sensitivity and also keeps specificity.
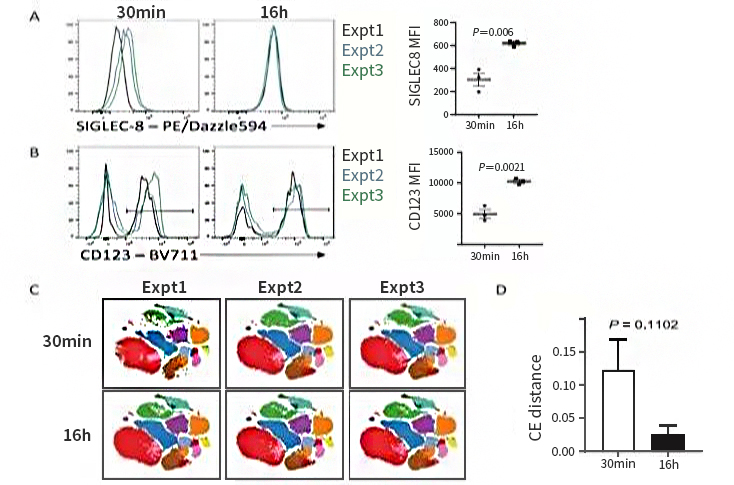
4. Decrease of Result Variability and Batch Effects
Extended antibody duration can decrease batch-to-batch variability. Antigen-antibody binding reaction is reversible. In shorter incubation, binding rate is faster than dissociation rate. Slight time differences easily result in fluorescent signal fluctuation and affect quantitative accuracy. Extension to 16~20h can balance the reaction and stabilize the signal. Overnight staining with standardized operation can obviously decrease Inter-CV to improve reproducibility. Compared with 30min staining data, overnight staining decreases variability and improves resolution. t-SNE analysis also shows batch effects are less.
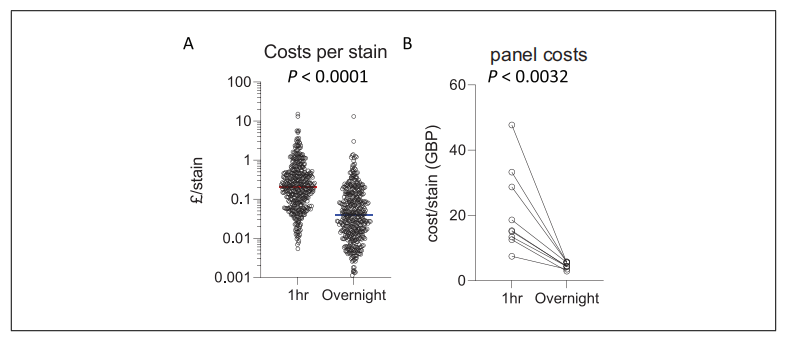
5. Saving Cost via Longer Incubation Duration
Roles of antibody titration in successful flow cytometry are very important. Lower antibody concentration can result in indistinguishable positive and negative signals. Higher concentration may cause non-specific binding and signal shift of negative population, affecting results analysis. Thus, antibody concentration should be optimized.
Antibodies used in overnight staining are less than 30min staining, decreasing from 5 to 100 fold. However, clear signal can be still obtained to improve sensitivity and specificity.
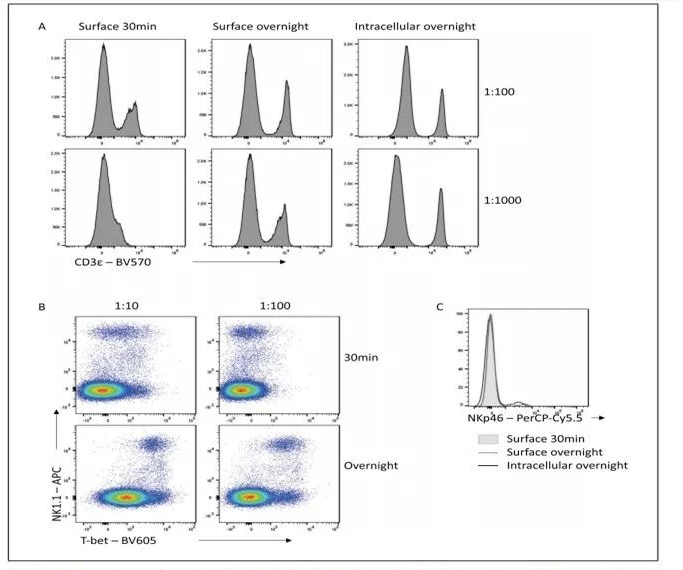
6. Flexibility of Panel Design
In multicolor flow cytometry panel design, suboptimal fluoresceins - labeled antibodies affect detection effects. Fixation and overnight staining can improve signal-to-noise ratio, resolution and panel flexibility. E.g. It's difficult for CD3-BV570 to distinguish positive and negative population in 30min staining. Lower concentration in overnight staining can clearly separate. Similar situations are also found in Tbet-BV605 and NKp46-PerCP-Cy5.5. Extended incubation duration can help better antibody-antigenic peptide binding.
| Recommended Products | |||
| Species | Cell Populations | Flow Cytometry Antibody Combination | Cat.No |
| Human | T/B/NK cell populations detection | CD45-PerCP | PCP-30039 |
| CD3-FITC | FITC-30004 | ||
| CD16-PE | PE-30061 | ||
| CD56-PE | PE-30008 | ||
| CD19-APC | APC-30066 | ||
| Human | Thl/Th2 cell populations detection | CD3-PerCP/Cyanine5.5 | PCP55-30004 |
| CD4-FITC | FITC-30005 | ||
| IFN-γ-PE | PE-30053 | ||
| IL4-APC | APC-30043 | ||
| Mouse | Thl/Th2 cell populations detection | CD3-PerCP/Cyanine5.5 | PCP55-30002 |
| CD4-FITC | FITC-30128 | ||
| IFN-γ-PE | PE-30074 | ||
| IL4-APC | APC-30026 | ||
| Human | Treg cell populations detection | CD4-FITC | FITC-30005 |
| CD25-PE | PE-30035 | ||
| CD3-PerCP-Cy5.5 | PCP55-30004 | ||
| CD127-FineTest®647 | F647-30033 | ||
| Mouse | Treg cell populations detection | CD4-FITC | FITC-30128 |
| CD25-APC | APC-30017 | ||
| FOXP3-PE | PE-30111 | ||
REFERENCES
[1]Do more with Less: Improving High Parameter Cytometry Through Overnight Staining, PMID: 36373983.
[2]Optimization of a 40-Color Spectral Flow Cytometry Panel for Human Blood Immunophenotyping: Impact of Blood Volume, Sample Preservation, and Overnight Staining, PMID: 41454662.
