Abstract: Flow cytometry is the high-throughput and multi-parameter single-cell analysis technology based on fluorescence labeling and laser detection. Roles and applications in life science research and clinical diagnosis are very important. This technology quantitatively analyzes the phenotype, function and molecular level of a large number of cells. Flow Cytometry applications are specified in detail below, covering methodology, detection content, results interpretation and scientific significance etc.
Keywords: Flow Cytometry Applications, Results Interpretation, Flow Cytometry Analysis
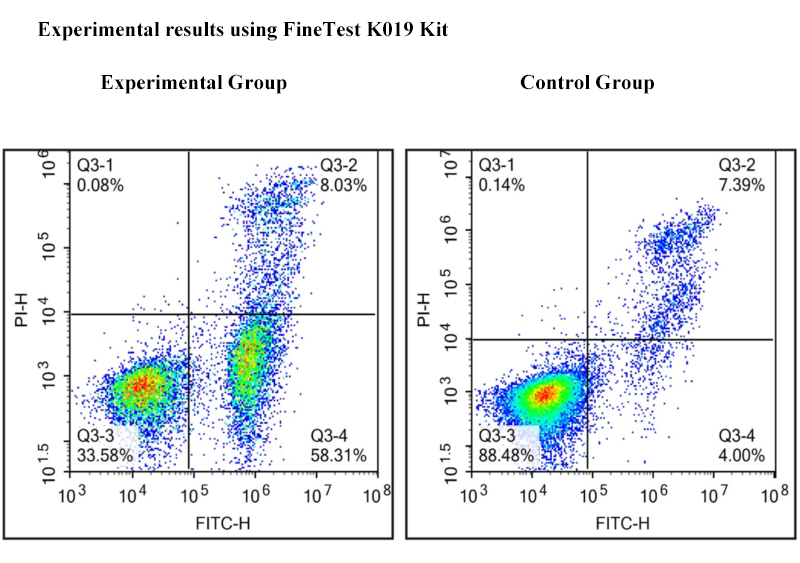
1. Immunophenotyping Analysis
Methodology: Detect with multi-color fluorescent-labeled monoclonal antibodies and flow cytometry. Synchronously analyze the surface of various cells or intracellular antigens.
Detection Content: Recognize cell markers(e.g. CD3, CD4, CD8, CD19, CD34 etc) with fluorescent antibodies. Accurately analyze proportion and phenotype of immunocyte subgroups(e.g. T cell, B cell, NK cell etc).
Results Interpretation: Abnormal proportion of subgroups(e.g. imbalance of CD4+/CD8+) indicates immunologic dysfunction, helpfully judging infection or autoimmune disease. Specific antigen expression(e.g. CD34, CD19) assists in tumor immunophenotyping and MRD detection.
Applications: Widely applied in immunological research, hematological malignancy diagnosis and immune function monitoring after transplantation.
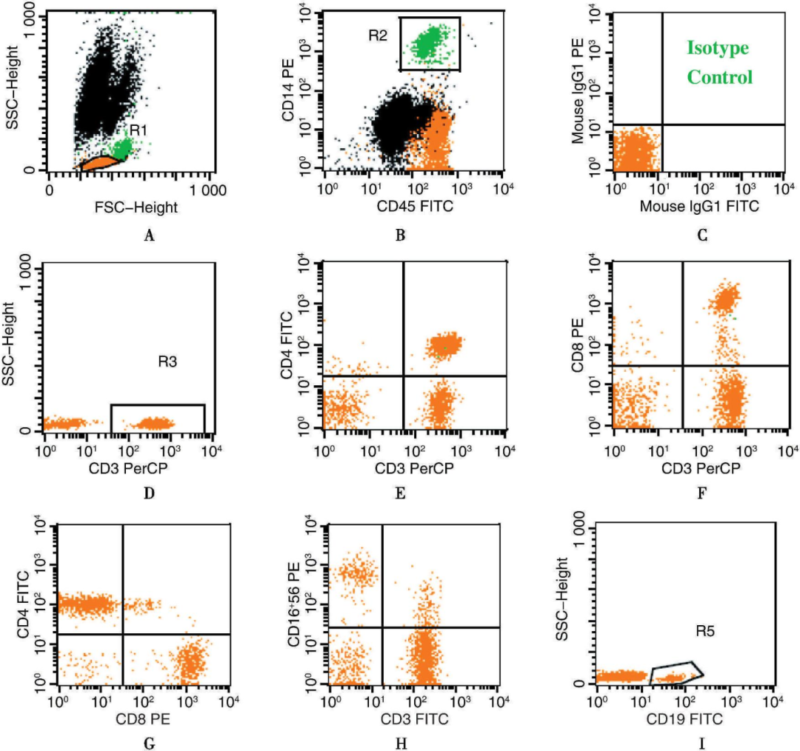
2. FACS Cell Cycle Analysis
Methodology: Detect with DNA-binding fluorescent dyes(e.g. PI, DAPI) and proliferation-associated protein Ki67. Alternatively, track cell division with CFSE dye.
Detection Content: Judge cell cycle distribution in each phase(e.g. G0/G1, S, G2/M) via measuring cellular DNA content. Alternatively, analyze cell division times via CFSE fluorescent changes.
Results Interpretation: Increase of cell proportion in G1 phase indicates cycle arrest or DNA damage repair. Increased S phase shows enhanced cell proliferation. CFSE fluorescent decrement shows increased division times. Evaluate cell activity and drugs/factor-stimulated effects.
Applications: Widely applied in tumor proliferation, drug activity screening and analysis of stem cell proliferation.
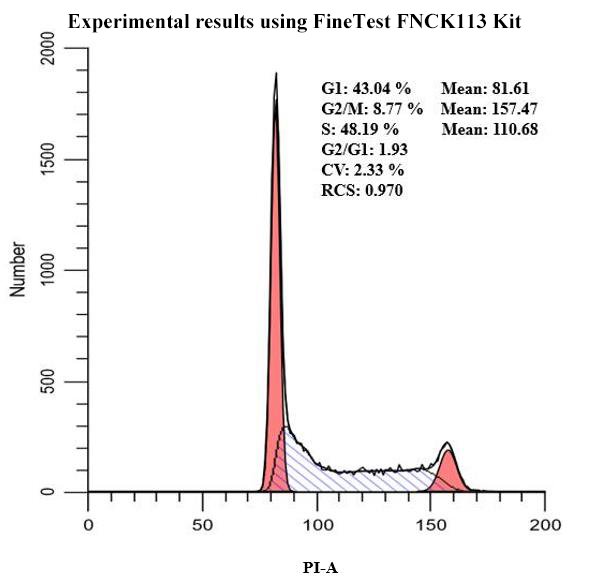
3. Apoptosis and Necrosis Detection
Methodology: Distinguish apoptosis and necrosis via fluorescent-labeling, using Annexin V-FITC/PI double staining method.
Detection Content: Annexin V recognizes exposed phosphatidylserine of early apoptotic cells. PI enters the damaged membrane of late apoptotic or necrotic cells.
Results Interpretation: Annexin V+/PI− shows early apoptosis. Annexin V+/PI+ shows late apoptosis or necrosis. Evaluate drug or radiation induced cytotoxicity. Analyze apoptosis pathway further via JC-1 or Caspase activity.
Applications: Applied in research on tumor therapy mechanism and analysis of drug toxicology.
4. Cell Function Assay
Methodology: Detect calcium flux with calcium fluorescent probe(e.g. Fluo-3/Fluo-4). Evaluate phagocytosis with fluorescent microspheres or fluorescent-labeled bacteria. Analyze secretion of multi-factors via intracellular staining or cytometric bead array(CBA).
Detection Content: Changes in calcium signaling show the activation process of immune cells. Phagocytosis evaluates the activity of innate immune cells(e.g. macrophages). Detect cytokines(e.g. IFN-γ, IL-4) for balance analysis of Th1/Th2.
Applications: Widely applied in research on immune regulation mechanism, analysis of infected immune response and development of new vaccines.
5. FACS Single Cell Sorting and Protein Engineering
Methodology: Use FACS technology to accurately sort the target cell via fluorescent labeling and scattering feature.
Detection Content: Sort the specific cell group according to cell surface markers or fluorescent signaling(e.g. CD34+ stem cell, CAR-T cell). Alternatively, sort bacteria with the target gene for cloning.
Results Interpretation: FACS can achieve stem cell purification for transplantation therapy. Alternatively sort highly expressed target protein for cloning cells to improve screening efficiency of antibody drugs.
Applications: Widely applied in regenerative medicine, synthetic biology and biopharmaceuticals.
6. Flow Cytometry Bacteria Detection
Methodology: Analyze with fluorescent dye labeling and scattering signal. Rapidly detect and quantitatively evaluate microorganisms.
Detection Content: Analyze the number, active state and metabolic features of bacteria in the water or fermentation broth. Distinguish viable and dead bacteria via SYTO9/PI staining.
Results Interpretation: The ratio of viable and dead bacteria can evaluate sterilization or disinfection effects. Analysis for microbial community structure can help to understand environmental change and microecology balance.
Applications: Widely applied in environmental monitoring, food safety detection and quality control for industrial fermentation.
7. Clinical Applications of Flow Cytometry
Methodology: Analyze expression level of immune checkpoint molecules(e.g. PD-1, PD-L1) via multi-parameter flow cytometry. Comprehensively evaluate via phenotype and functional markers.
Detection Content: Analyze expression of PD-1/PD-L1 in tumor infiltrating lymphocytes(TILs). Evaluate activity and function of T cell.
Results Interpretation: High expression of PD-1 shows exhausted state of T cells, assisting in judging whether patients are suitable for checkpoint inhibitor therapy. Monitor expansion and persistence of CAR-T cell. Provide evidences to immunotherapy optimization.
Applications: Widely applied in evaluating tumor immunotherapy and individual treatment planning.
Flow cytometry promotes the conversion of fundamental research to clinical applications, covering tumor diagnosis and drug development. The development of mass spectrometry flow cytometry and microfluidic technology can further analyze more complex biosystem.
| Recommended Products | |||
| Species | Cell Populations | Flow Cytometry Antibody Combination | Cat.No |
| Human | T/B/NK cell populations detection | CD45-PerCP | PCP-30039 |
| CD3-FITC | FITC-30004 | ||
| CD16-PE | PE-30061 | ||
| CD56-PE | PE-30008 | ||
| CD19-APC | APC-30066 | ||
| Human | Thl/Th2 cell populations detection | CD3-PerCP/Cyanine5.5 | PCP55-30004 |
| CD4-FITC | FITC-30005 | ||
| IFN-γ-PE | PE-30053 | ||
| IL4-APC | APC-30043 | ||
| Mouse | Thl/Th2 cell populations detection | CD3-PerCP/Cyanine5.5 | PCP55-30002 |
| CD4-FITC | FITC-30128 | ||
| IFN-γ-PE | PE-30074 | ||
| IL4-APC | APC-30026 | ||
| Human | Treg cell populations detection | CD4-FITC | FITC-30005 |
| CD25-PE | PE-30035 | ||
| CD3-PerCP-Cy5.5 | PCP55-30004 | ||
| CD127-FineTest®647 | F647-30033 | ||
| Mouse | Treg cell populations detection | CD4-FITC | FITC-30128 |
| CD25-APC | APC-30017 | ||
| FOXP3-PE | PE-30111 | ||
REFERENCES
[1]Flow Cytometry Multiplex Bead Array Technology and Its Immunological Clinical Applications in Covid-19 Era, PMID: 41200584.
[2]A User-Centric Approach to Reliable Automated Flow Cytometry Data Analysis for Biomedical Applications, PMID: 40001293.
