During flow cytometry, multicolor antibody staining is the common requirement. Some multicolor flow cytometry protocols should be followed during color matching.
1. Strongly/Weakly Expressed Antigens
Weakly expressed antigens match with strong fluorochromes. Strongly expressed antigens match with weak fluorochromes. This matching protocol helps to improve signal-to-noise ratio. Then, the signal of weakly expressed antigens will not be covered by strongly expressed antigens.
Applications: During antibody selection, suitable fluorochromes depend on antigen expression. E.g. for strongly expressed antigen CD45, weaker fluorochrome like FITC is proper. For weakly expressed antigens(e.g. some cytokine receptors), stronger fluorochrome like APC may be required.
2. Avoid Fluorescent Leak
Co-expressed antigens: For antigens co-expressed on the same cell, avoid to use interfered fluorochromes. Fluorochrome combinations with less spectral overlap are required. In the figure below, CD25 and Foxp3 antigens are co-expressed antigens.

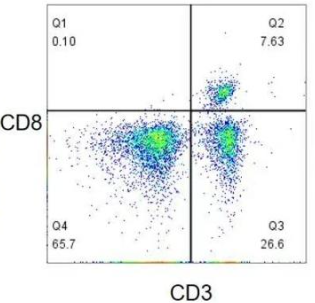
Mutually exclusive antigens refer to antigens won't be co-expressed on the same cell. In the figure below, CD4 and CD8 are not co-expressed on the same cell, allowing slight fluorescent leak which has no interference on another antigen detection.

Maternal and offspring antigens: allow the leak of offspring(e.g. CD8) to maternity(e.g. CD3).
3. Instrument Configuration and Fluorochrome Selection
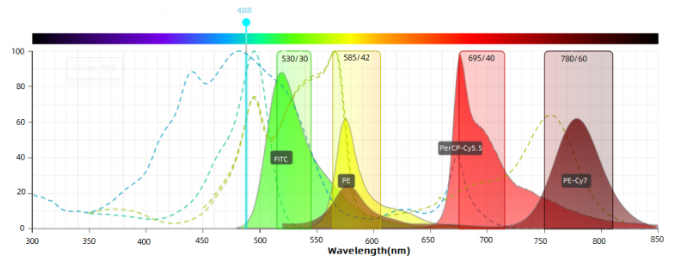
Channel Configuration: Before antibody color matching, the channel configuration of flow cytometer should be known, including the wavelength of laser, filter setting etc. Then, suitable fluorochromes can be selected to assure the accurate detection.
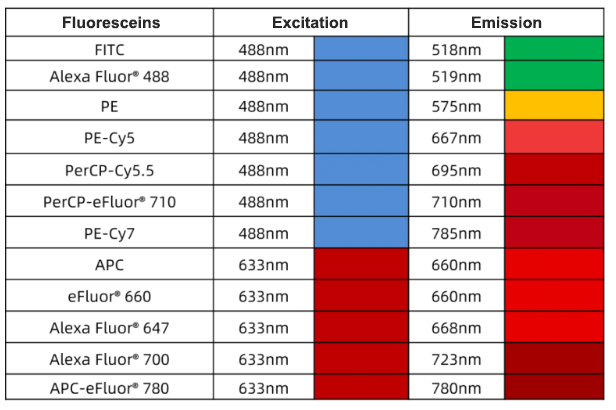
Fluoresceins can be divided into 4 types following excitation wavelength: 375 nm, 405 nm, 488 nm and 633 nm. Common excitation wavelengths are 488 nm and 633 nm fluoresceins, e.g. FITC, PE, APC, PI etc.
Fluorochrome Selection: Suitable fluorochromes depend on channel configuration and fluorochrome features(e.g. excitation spectrum, emission spectrum, brightness etc). Usually, each fluorochrome has the specific excitation and emission wavelength range. Make sure these wavelength ranges match with the channel configuration.
Fluorescein brightness matching: High brightness fluoresceins are recommended to antigens with low expression level. Highly expressed antigens use low brightness fluoresceins.

Minimal spectral overlap among fluoresceins: choose fluorescein combinations with less spectral overlap, e.g. FITC/PE-Cy7; choose different laser-excited fluorescein combinations: e.g. FITC/APC, PE/APC.
4. Standardized Operation and Quality Control
Standardized Operation: During multicolor antibody staining, following standardized operations can decrease experimental error and improve data reliability, including cell processing, antibody incubation and washing etc.
Quality Control: During the experiment, quality control is required to assure the accurate data. E.g. set negative and positive control to monitor the specificity and sensitivity. Meanwhile, remove interferences between different channels with fluorescence compensation.
5. Notes
Avoid to use composite fluorochromes: Although composite fluorochromes can offer multicolor choices, they are easily broken and degrade to cause unstable experiment results.
Consider autofluorescence: some cells have higher autofluorescence level and may interfere with fluorochrome detection. During choosing fluorochromes, consider the autofluorescence feature of cells and choose suitable excitation and emission wavelength range to avoid the interference of autofluorescence.
6. Conclusion
Above all, color matching of flow cytometry antibody is a complex and precise process. We need to consider antigen expression, fluorochrome features, instrument configuration and experimental operations etc. Following multicolor flow cytometry protocols and notes above can assure the accuracy and reliability of multicolor antibody staining.
7. Recommended Products
|
Species |
Cell Populations |
Flow Cytometry Antibody Combination |
Cat.No |
|
Human |
T/B/NK cell populations detection |
CD45-PerCP CD3-FITC CD16-PE CD56-PE CD19-APC |
|
|
Human |
Th1/Th2 cell populations detection |
CD3PerCP/ Cyanine5.5 CD4-FITC IFN-γ-PE IL4-APC |
|
|
Mouse |
Th1/Th2 cell populations detection |
CD3-PerCP/ Cyanine5.5 CD4-FITC IFN-γ-PE IL4-APC |
|
|
Human |
Treg cell populations detection |
CD4-FITC CD25-PE CD127-APC |
|
|
Mouse |
Treg cell populations detection |
CD4-FITC CD25-PE FOXP3-APC |
|
Isotype Control Antibodies
|
Cat.No |
Product Name |
Host |
Marker |
Size |
|
|
APC Mouse IgG2b,κ Isotype Control(MPC-11) |
Mouse |
APC |
20T/100T |
|
|
PE Mouse IgG2b,κ Isotype Control(MPC-11) |
Mouse |
PE |
20T/100T |
|
|
FITC Mouse IgG2b,κ Isotype Control(MPC-11) |
Mouse |
FITC |
20T/100T |
|
|
PE Mouse IgG2a,κ Isotype Control(C1.18.4) |
Mouse |
APC |
20T/100T |
|
|
Mouse Anti-MPV IgG ELISA Kit |
Mouse |
PE |
20T/100T |
|
|
PerCP Mouse IgG1,κ Isotype Control(MOPC-21) |
Mouse |
FITC |
20T/100T |
|
|
APC Mouse IgG1,κ Isotype Control(MOPC-21) |
Mouse |
PerCP |
20T/100T |
|
|
PE Mouse IgG1,κ Isotype Control(MOPC-21) |
Mouse |
APC |
20T/100T |
|
|
FITC Mouse IgG1,κ Isotype Control(MOPC-21) |
Mouse |
PE |
20T/100T |
|
|
APC Rat IgG1,κ Isotype Control(HRPN) |
Mouse |
FITC |
20T/100T |
|
|
PE Rat IgG1,κ Isotype Control(HRPN) |
Rat |
APC |
20T/100T |
|
|
Porcine Anti-MPV IgG ELISA Kit |
Rat |
PE |
20T/100T |
|
|
FITC Rat IgG1,κ Isotype Control(HRPN) |
Rat |
FITC |
20T/100T |
|
|
APC Rat IgG2a,κ Isotype Control(2A3) |
Rat |
APC |
20T/100T |
|
|
PE Rat IgG2a,κ Isotype Control(2A3) |
Rat |
PE |
20T/100T |
|
|
FITC Rat IgG2a,κ Isotype Control(2A3) |
Rat |
FITC |
20T/100T |
REFERENCES
[1]Functional analysis of human NK cells by flow cytometry, PMID: 20033652.
[2]Flow cytometric analysis of T lymphocyte proliferation in vivo by EdU incorporation, PMID: 27816727.
