Abstract: Ivonescimab(also called AK112 or SMT112) is the initiated humanized bispecific antibody against PD-1 and VEGF-A. It can bind with VEGF-A and PD-1 at the same time, competitively preventing their interaction with ligand. AK112 achieves one antibody with double targets and improves antitumor activity to provide a new treatment plan for patients. In clinical trials, treatment-related adverse events(TRAE) or immune-related adverse events(irAE) caused by AK112 is not observed. Overall, AK112 is found to be well-tolerated for the treatment of NSCLC. The TRAE incidence is lower than combined chemotherapy, which provides a potential treatment plan without chemotherapy in the future.
Keywords: Bispecific Antibody, Cancer Immunotherapy, Antibody Drug
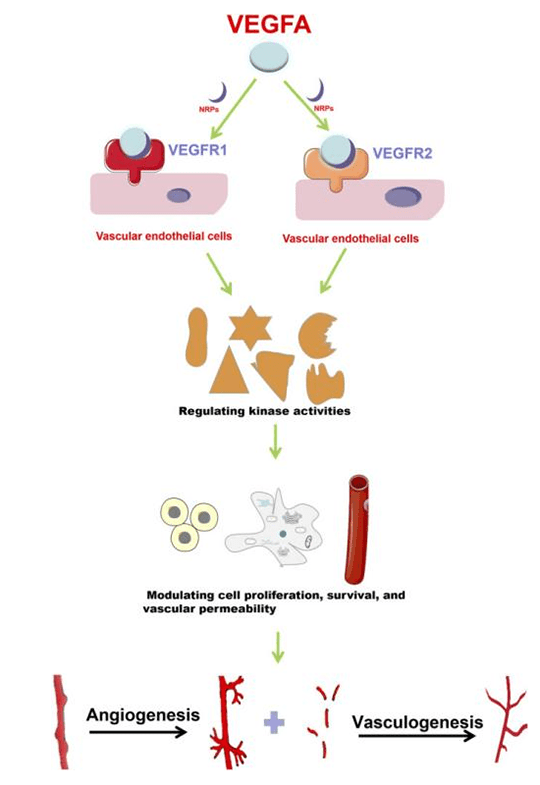
1. VEGF-A Protein
VEGF-A is found in various variants. VEGFA165 is the most common one. These variants play different roles by binding with different VEGF receptors(e.g. VEGFR1, VEGFR2, VEGFR3 etc.). Receptor tyrosine kinase(RTK) is the classical VEGF receptor. VEGFR1 and VEGFR2 are mainly expressed in vascular endothelial cell(ECs). VEGFR3 is mainly distributed in lymphatic ECs to regulate generation of lymphatic vessels. VEGFA can bind with VEGFR1 or VEGFR2 according to cell type and specific functions. Compared with VEGFR2, VEGFR1 has a higher binding affinity for VEGFA. But the activity of tyrosine phosphorylation is weaker. VEGFR2 plays an important role in VEGF mediated angiogenesis and VEGF signal transduction in ECs.
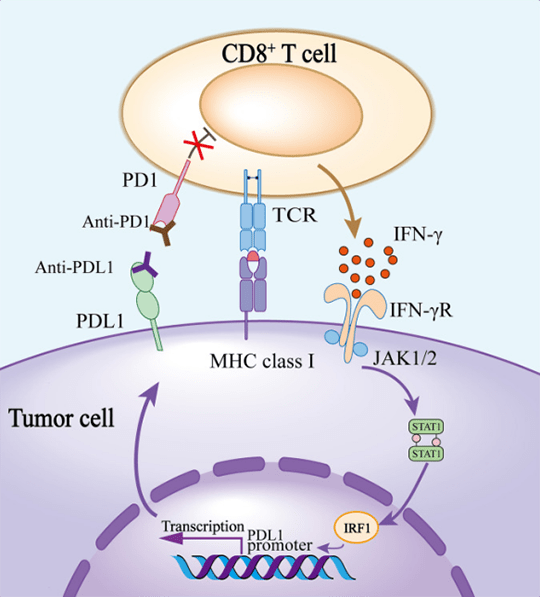
2. Difference between PD-1 and PD-L1
PD-1 protein(also called CD279) consists of 288 amino acids, and exists on the surface B cell, T cell and natural killer cell. It's up-regulated by various inflammatory stimulations in dendritic cell(DCs). PD-L1 is usually expressed by macrophage, some activated T cell and B cell, DCs, and some epithelial cells. Antigen-specific T cell plays an important role in eliminating tumor cells. Once tumor cells release tumor antigens, cancer immunity cycle will be triggered. Antigen-specific T cell initially recognizes major histocompatibility complex(MHC) presented tumor antigens. After activation and proliferation, T cell goes to the specific site along concentration gradient of chemokine. When targeting the same antigen on MHC, T cell releases IFN-γ to improve tumor killing effect. Meanwhile, The signal of TCR up-regulates the expression of PD1 on the surface of T cell. The binding between PD1 and PDL1 negatively regulates and weakens the anti-tumor function of T cell.
3. Anti-VEGF Drugs
VEGF is widely expressed in human tumor and cells. Especially, the subtype VEGF165 and VEGF121 are very common, which are closely related to development, metastasis, pathologic grading and prognosis of lung cancer, thyroid cancer, breast cancer, hemangioma, central nervous system tumor etc. There are five kinds of globally approved VEGF/VEGFR macromolecular drugs, including three monoclonal antibodies: Bevacizumab, Ramucirumab, Ranibizumab; and two fusion proteins: Aflibercept, Conbercept.
4. Recommended Products
4.1. Protein
|
Cat.No |
Product Name |
MW(kDa) |
Host |
|
Recombinant Human VEGFA |
15.9 |
E.Coli |
|
|
Recombinant Human CD279 |
41.1 |
HEK293 cells |
|
|
Recombinant Human CD274 |
35-38 |
HEK293 cells |
4.2. Antibody
|
Cat.No |
Product Name |
MW(kDa) |
Type |
|
VEGF antibody |
45 |
mAb |
|
|
PD-1 antibody |
32, 50 |
mAb |
|
|
CD274 antibody |
50, 70 |
pAb |
4.3. ELISA Kit
|
Cat.No |
Product Name |
Range |
Sensitivity |
|
Human VEGF-A ELISA Kit |
31.25-2000pg/ml |
18.75pg/ml |
|
|
Bevacizumab(Avastin) ELISA Kit |
0.313-20ng/ml |
0.188ng/ml |
|
|
Human PD1/PDL1 Inhibitor Screening Assay Kit |
31.25-2000pg/ml |
18.75pg/ml |
|
|
Human PD-1/PDCD1 ELISA Kit |
31.25-2000pg/ml |
18.75pg/ml |
|
|
Anti-Human VEGF ELISA Kit |
0.312-20ng/ml |
0.188ng/ml |
|
|
Human anti-Bevacizumab(Avastin) antibody ELISA Kit |
0.312-20ng/ml |
0.188ng/ml |
REFERENCES
[1]Cadonilimab, a tetravalent PD-1/CTLA-4 bispecific antibody with trans-binding and enhanced target binding avidity, PMID: 36872527.
[2]Synergistic efficacy of simultaneous anti-TGF-β/VEGF bispecific antibody and PD-1 blockade in cancer therapy, PMID: 37573354.
