FineTest ELISA kit contributes to the research on metastatic breast cancer. The immunoassay is designed to measure CCL6 level in tissue lysates.
Article Title: The Ly6ghigh neutrophil subset dictates breast cancer lung metastasis via CD8+ T cell death
Journal Title: Cancer Communications
DOI: 10.34133/cancomm.0003
IF: 24.9
Abstract: Background: Lung metastasis is a leading cause of breast cancer (BC)-related mortality, driven by the immunosuppressive traits of the metastatic tumor microenvironment. However, the mechanisms underlying cell-cell crosstalk in shaping immune evasion within the metastatic niche remain poorly defined. Neutrophil extracellular traps (NETs) and their associated proteins, such as cathelicidin, have emerged as key mediators of metastatic regulation in cancer. Here, we aimed to decipher the interaction between a neutrophil subset characterized by high expression of lymphocyte antigen 6 complex locus g (Ly6ghigh) and cluster of differentiation 8- positive T lymphocytes (CD8+ T cells), mediated via cathelicidin embedded in NETs, as well as their synergistic mechanism and cooperative role in promoting lung metastasis of BC. Methods: We characterized neutrophil heterogeneity and functional dynamics by performing single-cell RNA sequencing and flow cytometry on lung tissues derived from murine models of BC lung metastasis. We utilized Cathelicidin-related antimicrobial peptide (Cramp) knockout mice to dissect the role of cathelicidin in NETs. The spatial co-localization of apoptotic CD8⁺ T cells and NETs was analyzed using multiplex immunofluorescence, and the molecular interactions were probed by protein binding assays. Results: Neutrophils in the lung metastatic niche were classified into two subsets based on the Ly6g expression: Ly6ghigh and Ly6glow neutrophils. Ly6glow neutrophils, which were recruited in the macro-metastatic stage, exhibited myeloid-derived suppressor cell-like characteristics. Notably, Ly6ghigh neutrophils induced CD8⁺ T cell apoptosis through NET formation, with apoptotic CD8⁺ T cells spatially clustered within NETrich areas. Mechanistically, NET-derived cathelicidin (Cramp in mice) directly bound to mitochondrial adenine nucleotide translocator 1 (Ant1) in CD8⁺ T cells, triggering conformational changes and complex formation with voltage-dependent anion channel 1 (Vdac1). These events resulted in the opening of the mitochondrial permeability transition pore and loss of mitochondrial membrane potential. Conclusions: Our study demonstrates that Ly6ghigh neutrophils play a critical role in immunosuppression and immune evasion through NET-induced apoptosis of CD8+ T cells. These findings underscore the importance of NETs and cathelicidin in BC lung metastasis, suggesting their potential as therapeutic targets in restoring anti-tumor immunity and in preventing metastatic progression.
Keywords: pulmonary Ly6ghigh neutrophils, CD8+ T cell, metastasis niche, NETs, cathelicidin
Immunoassay
| FineTest Product | Sample | Species | Detection Target |
| Mouse CCL6(C-C motif chemokine 6) ELISA Kit(EM1474) | tissue lysates | Mouse | CCL6 |
Validated Image
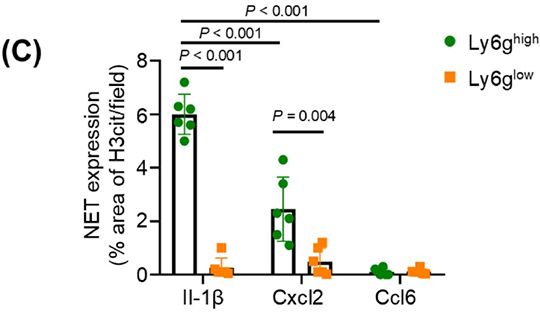
Figure Source: Cancer Communications, 2025 Dec 15; 2523-3548. doi: 10.34133/cancomm.0003.
