FineTest ELISA kit contributes to the research on immune response to COVID-19. The immunoassay is designed to measure IgG antibodies in plasma.
Article Title: Humoral and cellular immune responses in people living with HIV following successive COVID-19 vaccine booster doses
Journal Title: Clinical Microbiology and Infection
DOI: 10.1016/j.cmi.2025.06.007
IF: 10.9
PMID: 40513829
Abstract: Objective: The aim of this study was to evaluate humoral and cellular immune responses in people living with HIV (PLWH) following successive COVID-19 vaccine booster doses, to determine immune correlates associated with clinical outcomes (avoiding severe infections) and epidemiological impacts (preventing new infections), a topic of growing controversy in this vulnerable population. Methods: A prospective study followed 151 PLWH on suppressive antiretroviral therapy who completed initial COVID-19 vaccination and received two additional vaccine doses. The study evaluated changes in SARS-CoV-2-specific antibodies, SARS-CoV-2-ACE2 binding inhibition rates, spike-specific memory B cells, and CD4/CD8 cell responses to variants (Ancestral, Delta, and Omicron), considering initial vaccine type, prior infections, and levels of immunosuppression. Results: Vaccine doses progressively enhanced antibody levels, memory B cells, and T-cell responses. PLWH with CD4 counts ≤350 cells/mm3 showed impaired memory B cell production vs. those with CD4 >500 cells/mm3 after the third dose (0.39% [0.29-0.55] vs. 0.68% [0.49-0.86]; p < 0.001). Immune responses remained consistent across variants. Non-infected PLWH receiving plasmid vector vaccines demonstrated lower antibody levels against Delta and Omicron (10 930 ng/mL [9623-12 511] vs. 13 340 ng/mL [10 602-14 724], p 0.018; 399 ng/mL [335-702] vs. 615 ng/mL [492-924], p 0.041) compared with infected PLWH. IgA-producing memory B cells increased after the third booster, particularly with mRNA vaccines (0.05% [0.0-0.09] vs. 0.11 [0.07-0.17], p < 0.001 in non-infected individuals). Post-booster infection rates were higher in previously uninfected individuals (25% vs. 4% after the second booster, p < 0.001), especially among vector vaccine recipients (34.6% vs. 14.5%, p 0.028). Discussion: This study reveals that successive vaccine doses significantly enhance immune responses in PLWH, improving antibody levels, IgA+ memory B cells, and T-cell responses, thereby reducing the risk of severe infections and potentially new infections. Nevertheless, low CD4 counts result in reduced memory B cells, necessitating tailored vaccination strategies. mRNA vaccines also offer superior protection against breakthrough infections during variant surges.
Keywords: COVID-19 vaccine, HIV-1, Immune response, Immunosuppression, SARS-CoV-2
Immunoassay
| FineTest Product | Sample | Species | Detection Target |
| Human anti-SARS-CoV2(S1 protein) IgG ELISA Kit(EH4995) | plasma | Human | IgG(S1 protein) |
| Human anti-SARS-CoV2(S-RBD)(Delta B.1.617.2)IgG ELISA Kit(EH4994) | IgG(Delta B.1.617.2) | ||
| Human anti-SARS-CoV2(S-RBD)(Omicron,B.1.1.529) IgG ELISA Kit(EH4971) | IgG(Omicron,B.1.1.529) |
Validated Image
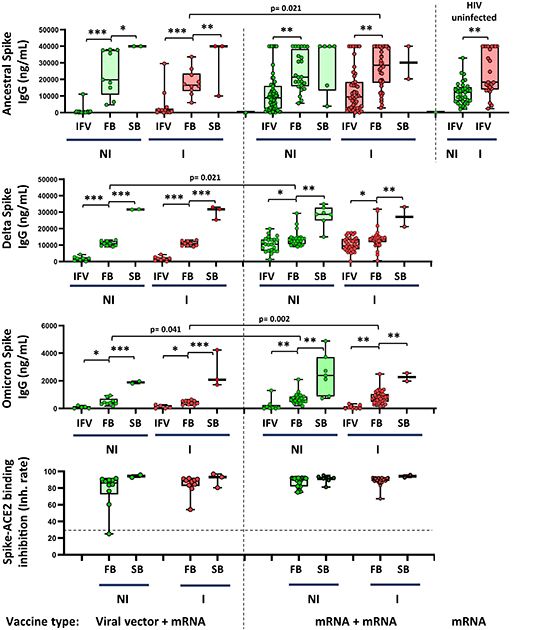
Figure Source: Clin Microbiol Infect. 2025 Jun 11:S1198-743X(25)00294-0. doi: 10.1016/j.cmi.2025.06.007.
