FineTest Elisa kit contributes to the research on essential thrombocythemia blood cancer. The immunoassay is designed to measure suPAR concentration in serum.
Publication Details
Article Title: Occurrence of a paroxysmal nocturnal hemoglobinuria clone in an essential thrombocythemia: a link between PIGV and MPL
Journal Title: Haematologica
DOI: 10.3324/haematol.2021.279804
IF: 11.04
PMID: 35081686
Abstract: Paroxysmal nocturnal hemoglobinuria (PNH) is a rare acquired hemopathy (about 1.3 individuals per million incidence) characterized by hemolytic anemia and venous thrombosis.1 The molecular defect involved is glycosylphosphatidylinositol (GPI) anchor loss related to X-linked PIGA gene mutations. Other genes responsible for the GPI anchor biosynthesis pathway could also be involved,2–5 and, research on their involvement in the pathophysiogenesis of PNH and its borderline forms is being uncovered. Here, we report a case of essential thrombocythemia (ET) caused by a somatic mutation in MPL (c.1544G>T:p.W515L) shortly preceded by copy-neutral loss of heterozygosity (CN-LOH) in cis on chromosome 1, leading to homozygosity of both the acquired MPL missense mutation and an inherited heterozygous stop-gain mutation in PIGV (c.1405C>T:p.R469X) causing PNH.
Keywords: Essential Thrombocythemia, Paroxysmal Nocturnal Hemoglobinuria, PNH Blood Disorder
Immunoassay
| FineTest Product | Sample | Detection Target | Species |
| Human suPAR(soluble urokinase-type plasminogen activator receptor) ELISA Kit (EH4026) | serum | suPAR | Human |
Validated Image
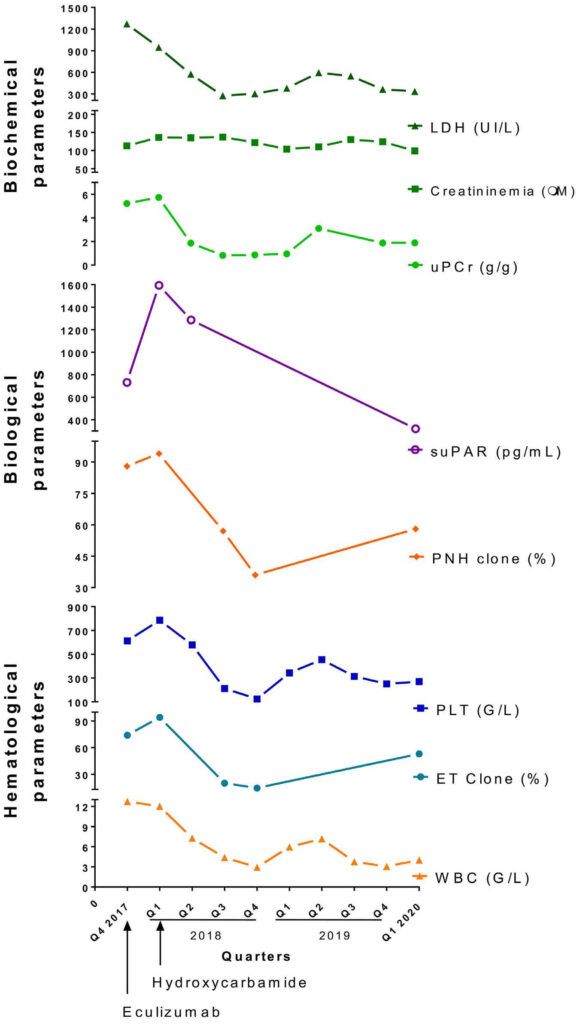
Figure Source: Haematologica. 2022 Aug 1;107(8):1989-1993. doi: 10.3324/haematol.2021.279804.
Figure 1. Follow-up over time of biochemical (LDH, creatininemia, proteinuria [urinary protein to creatinine ratio, uPCr], soluble urokinase-type plasminogen receptor [uPAR]) and hematological paroxysmal nocturnal hemoglobinuria (PNH) clone (flow cytometry), variant allele frequency (VAF) of the essential thrombocythemia (ET) clone, platelets (PLT), and leukocytes (white blood cells, WBC) parameters. Eculizumab treatment was first started in quarter 4 (Q4) of 2017, leading to a decrease in LDH Hydroxycarbamide was administered in Q1 of 2018. Apart from creatininemia, which did not vary, all other parameters followed the evolution of the PNH/ET clone undergoing cytoreductive treatment. The concentration of serum suPAR was assessed using the human suPAR enzyme-linked immunosorbant assay kit (Fine Test, EMELCA Biosciences) following the manufacturer's instructions.
