FineTest ELISA kit contributes to the research on pancreatic ductal adenocarcinoma. The immunoassay is designed to measure CTSA level in serum.
Article Title: Extracellular vesicles from obese visceral adipose promote pancreatic cancer development and resistance to immune checkpoint blockade therapy
Journal Title: Cell Metabolism
DOI: 10.1016/j.cmet.2025.10.015
IF: 30.9
PMID: 41338178
Abstract: Obesity is correlated with the development of multiple cancer types, and obese patients with pancreatic ductal adenocarcinoma (PDAC) show dismal prognosis and resistance to immune checkpoint blockade (ICB) therapy. The molecular mechanism is largely unknown. Here, we show that obese visceral adipose tissues (VATs) can communicate with distant PDAC by delivering extracellular vesicles (EVs) carrying signal molecules. We reveal that PDAC cells can take VAT-EVs into their lysosomes, where EV-delivered cathepsin A (Ctsa) stabilizes the ribonuclease Rnaset2b to produce free pseudouridine. Pseudouridine activates mast cells via increasing reactive oxygen species (ROSs) and decreasing H3K27me3 modification at the gene promoter. Activated mast cells inhibit CD8+ T cell activity, forming an immunosuppressive tumor microenvironment that enhances cancer progression. Animal experiments indicate that Ctsa knockdown effectively enhances ICB efficacy on PDAC. Our study uncovers a VAT-EV CTSA-pseudouridine-mast cell axis connecting obesity and cancer, which holds promise for developing new therapeutic strategies for obesity-related cancers.
Keywords: CSTA, mast cell, obesity, pancreatic cancer, pseudouridine, pancreatic ductal adenocarcinoma
Immunoassay
| FineTest Product | Sample | Species | Detection Target |
| Human CTSA(Lysosomal protective protein) ELISA Kit(EH1853) | serum | Human | CTSA |
Validated Image
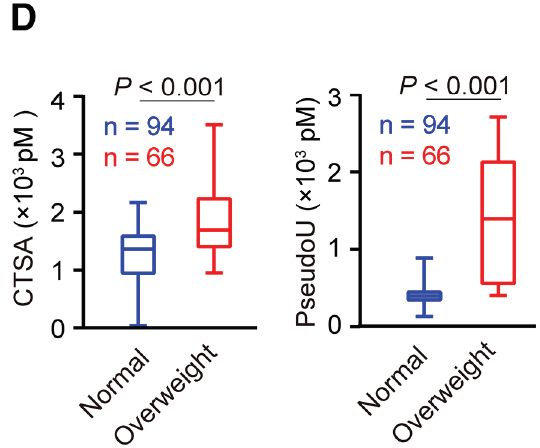
Figure Source: Cell Metab. 2025 Dec 2;37(12):2381-2401.e9. doi: 10.1016/j.cmet.2025.10.015.
