FineTest ELISA kit contributes to the research on cardiomegaly and heart failure. The immunoassay is designed to measure S100A8/A9 heterodimer level in plasma.
Article Title: Single-cell RNA sequencing reveals that myeloid S100A8/A9 is a novel regulator of the transition from adaptive hypertrophy to heart failure after pressure overload
Journal Title: Theranostics
DOI: 10.7150/thno.118369
IF: 13.3
PMID: 40860162
Abstract: Rationale: Infiltration of immune cells into the heart plays a crucial role in the transition from adaptive hypertrophy to heart failure (HF) following chronic pressure overload. However, the key factors in myeloid cells that regulate this process are still not well defined. Here, we studied the functional role of S100A8/A9 in myeloid cells during this transition. Methods: Cardiac hypertrophy and HF models were induced by transverse aortic constriction (TAC) for 1 to 4 weeks. The heterogeneity of CD45+ immune cells and the cellular sources of S100A8/A9 were analyzed using published single-cell RNA sequencing datasets. The effects of S100A8/A9 on TAC-induced hypertrophy and HF were verified in S100A9 knockout (KO) and bone marrow (BM)-chimeric mice and in an in vitro coculture system. Results: S100A8/A9 levels were significantly increased in HF patients and in TAC-induced HF model mice. Moreover, the TAC-induced transition from adaptive hypertrophy to HF was significantly attenuated in S100A9-KO mice and WT mice transplanted with S100A9-KO BM cells. Mechanistically, TAC-stimulated upregulation of S100A8/A9 in neutrophils induced an early inflammatory response and adaptive hypertrophy through activation of the p38 MAPK/JNK/AP-1 pathway, leading to increased production of IL-1β and chemokines (CCL2 and CCL6). These chemokines promoted the infiltration of CCR2+ macrophages to the damaged heart. Therefore, they exhibited upregulation of S100A8/A9, which led to exacerbation of inflammation, cardiac hypertrophy and fibrosis via activation of the NF-κB/NLRP3, AKT/Calcineurin A and TGF-β/Smad2 signaling pathways. Additionally, treating WT mice with the S100A9 inhibitor ABR-238901 prevented TAC-induced cardiac hypertrophy-related dysfunction. Conclusion: The present findings establish an S100A8/A9-related axis between myeloid cells and cardiac cells that drives the pressure overload-induced transition from hypertrophy to HF, suggesting that S100A8/A9 is a promising therapeutic target for this disease.
Keywords: S100A8/A9, cardiac hypertrophy, heart failure, inflammation, macrophages, neutrophils, cardiomegaly
Immunoassay
| FineTest Product | Sample | Species | Detection Target |
| Mouse calprotectin(S100A8/S100A9 Heterodimer)ELISA Kit(EM1620) | plasma | Mouse | calprotectin |
Validated Image
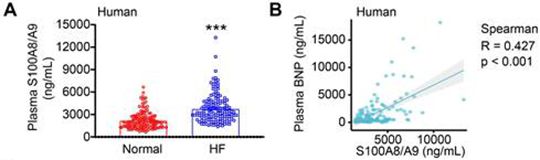
Figure Source: Theranostics. 2025 Jul 28;15(16):8587-8608. doi: 10.7150/thno.118369.
