FineTest ELISA kit contributes to the research on type 2 diabetes with peripheral angiopathy. The immunoassay is designed to measure lipoprotein (a) level in serum.
Article Title: Prognostic value of lipoprotein (a) for cardiovascular events after lower limb revascularization in diabetic patients with chronic limb-threatening ischemia
Journal Title: Cardiovascular Diabetology
DOI: 10.1186/s12933-025-02833-2
IF: 10.6
PMID: 40640828
Abstract: Background: Chronic limb-threatening ischemia (CLTI) presents a major clinical challenge in patients with Type 2 Diabetes Mellitus (T2DM), requiring lower extremity revascularization (LER) to mitigate adverse cardiovascular and limb outcomes. Lipoprotein(a) (Lp(a)) has been implicated in cardiovascular risk, but its role in patients with T2DM and CLTI undergoing revascularization remains unclear. Thus, this study aimed to investigate the prognostic value of Lp(a) levels in diabetic CLTI patients for major adverse cardiovascular events (MACE), major adverse limb events (MALE), or both after LER. Methods: In this prospective cohort study of 158 individuals with T2DM and CLTI undergoing LER, baseline clinical data were collected, including Lp(a) levels. Patients were followed for occurrence of MACE, MALE, or both over a 12-month period. Results: During follow-up, 74 patients (46.8%) experienced events (MACE, MALE, or both). Patients with events had significantly higher median Lp(a) levels than those without (48.0 vs. 8.1 mg/dL, p < 0.01). Lp(a) was independently associated with adverse events (HR 1.07, 95% CI 1.04-1.10; p < 0.01). In multivariable analysis, elevated Lp(a) was independently associated with both MACE (HR 1.08, 95% CI 1.03-1.13; p < 0.01) and MALE (HR 1.05, 95% CI 1.02-1.07; p < 0.01). An empirical Lp(a) cutoff of 29.6 mg/dL conferred a 3.8-fold increased risk of events (p < 0.01). Kaplan-Meier survival analysis further confirmed a significantly higher cumulative incidence of events in patients with Lp(a) levels above cutoff (p < 0.01). ROC curve comparison analysis showed that the inclusion of Lp(a) significantly improved the predictive performance of the base clinical model (AUC from 0.74 to 0.98, p < 0.01 for composite outcome; from 0.81 to 0.89, p = 0.03 for MACE; and from 0.78 to 0.92, p < 0.01for MALE). Conclusions: This study demonstrated that Lp(a) is a strong independent predictor of both cardiovascular and limb events in patients with T2DM undergoing LER for CLTI. These findings support the potential role of Lp(a) as a marker of residual risk in this high-risk population and suggest its utility in risk stratification.
Keywords: Lipoprotein(a) (Lp(a)), Major adverse cardiovascular events (MACE), Major adverse limb events (MALE), Peripheral artery disease (PAD), Peripheral Angiopathy
Immunoassay
| FineTest Product | Sample | Species | Detection Target |
| Human LPA(Lipoprotein a) ELISA Kit(EH0660) | serum | Human | LPA |
Validated Image
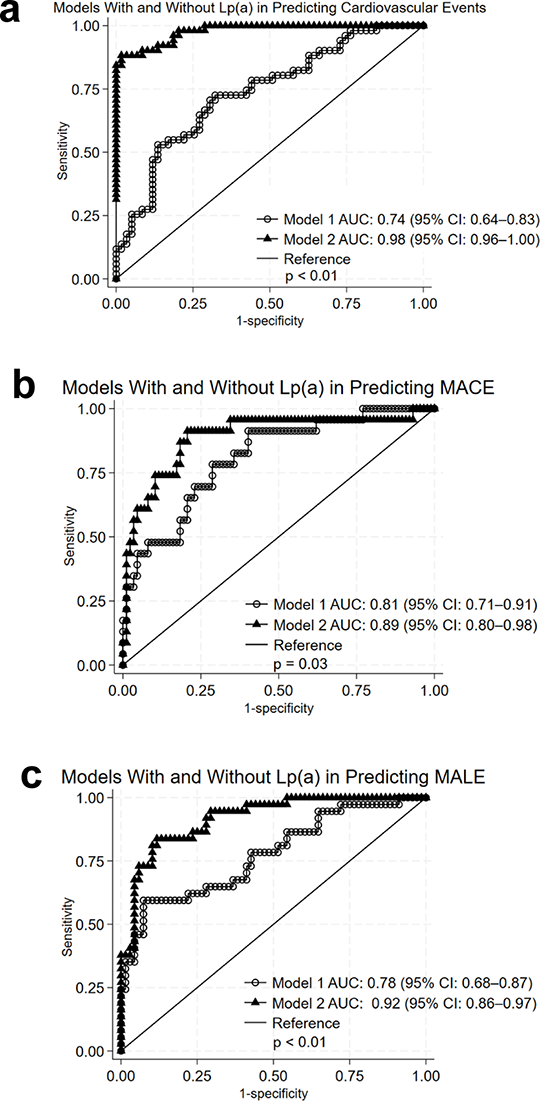
Figure Source: Cardiovasc Diabetol. 2025 Jul 10;24(1):271. doi: 10.1186/s12933-025-02833-2.
