Products
Mouse TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
This kit is based on Double antibody-Sandwich ELISA detection method and takes 4h assay time. The microplate provided in this kit has been precoated with anti TNF-α antibody. Add standard and properly diluted sample into relevant well respectively. After incubation, wash unbound components. Add biotinylated detection antibody. Then, it binds with TNF-α bound to precoated antibody. Wash unbound components and add HRP-Streptavidin Conjugate (SABC). Wash unbound components again and add TMB substrate solution. Then, TMB was catalyzed by HRP to produce a blue color product that turned yellow after adding a stop solution. Read the O.D. absorbance at 450nm in a microplate reader. Calculate the concentration of TNF-α in the sample by plotting standard curve. The concentration of the target substance is proportional to the OD450 value.
- Catalogue No.:
- EM0183
- Alias:
- TNFα ELISA Kit, Tumor Necrosis Factor Alpha ELISA Kit, TNF-α ELISA Kit, DIF ELISA Kit, TNF-alpha ELISA Kit, TNFA ELISA Kit, TNFSF2 ELISA Kit
- Species:
- Mouse
- Range:
- 3.906-250pg/ml
- Sensitivity:
- 2.344pg/ml
- SPECIFICATIONS
- CITATIONS
- Product Name
- Mouse TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Alias
- TNFα ELISA Kit, Tumor Necrosis Factor Alpha ELISA Kit, TNF-α ELISA Kit, DIF ELISA Kit, TNF-alpha ELISA Kit, TNFA ELISA Kit, TNFSF2 ELISA Kit
- Catalogue No.
- EM0183
- Size
- 48T/96T
- Species
- Mouse
- UniProt No.
- P06804
- Sample Type
- Serum, Plasma, Cell Culture Supernatant, cell or tissue lysate, Other liquid samples
- Detection Method
- Sandwich ELISA, Double Antibody
- Detection Wavelength
- OD450
- Reaction Duration
- 4 hours
- Range
- 3.906-250pg/ml
- Sensitivity
- 2.344pg/ml
- Storage
- 2-8°C(Sealed), Don't cryopreserve.
- Specificity
- Specifically binds with TNF-α , no obvious cross reaction with other analogues.
- ELISA Kit Components
Kit Components Item Size(48T) Size(96T) Storage Condition for Opened Kit E001 ELISA Microplate(Dismountable) 8×6 8×12 Put the rest strips into a sealed foil bag with the desiccant. Stored for 1 month at 2-8°C; Stored for 6 month at -20°C E002 Lyophilized Standard 1vial 2vial Put the rest standards into a desiccant bag. Stored for 1 month at 2-8°C; Stored for 6 month at -20°C E003 Biotin-labeled Antibody(Concentrated, 100X) 60ul 120ul 2-8°C (Avoid Direct Light) E034 HRP-Streptavidin Conjugate(SABC, 100X) 60ul 120ul E024 TMB Substrate 5ml 10ml E039 Sample Dilution Buffer 10ml 20ml 2-8°C E040 Antibody Dilution Buffer 5ml 10ml E049 SABC Dilution Buffer 5ml 10ml E026 Stop Solution 5ml 10ml E038 Wash Buffer(Concentrated, 25X) 15ml 30ml E006 Plate Sealer 3 pieces 5 pieces E007 Product Description 1 copy 1 copy - Required Instruments and Reagents
-
- Microplate reader (wavelength: 450nm)
- 37°C incubator (CO2 incubator for cell culture is not recommenced.)
- Automated plate washer or multi-channel pipette/5ml pipettor (for manual washing purpose)
- Precision single (0.5-10μL, 5-50μL, 20-200μL, 200-1000μL) and multi-channel pipette with disposable tips(Calibration is required before use.)
- Sterile tubes and Eppendorf tubes with disposable tips
- Absorbent paper and loading slot
- Deionized or distilled water
- Assay Procedure Summary
-
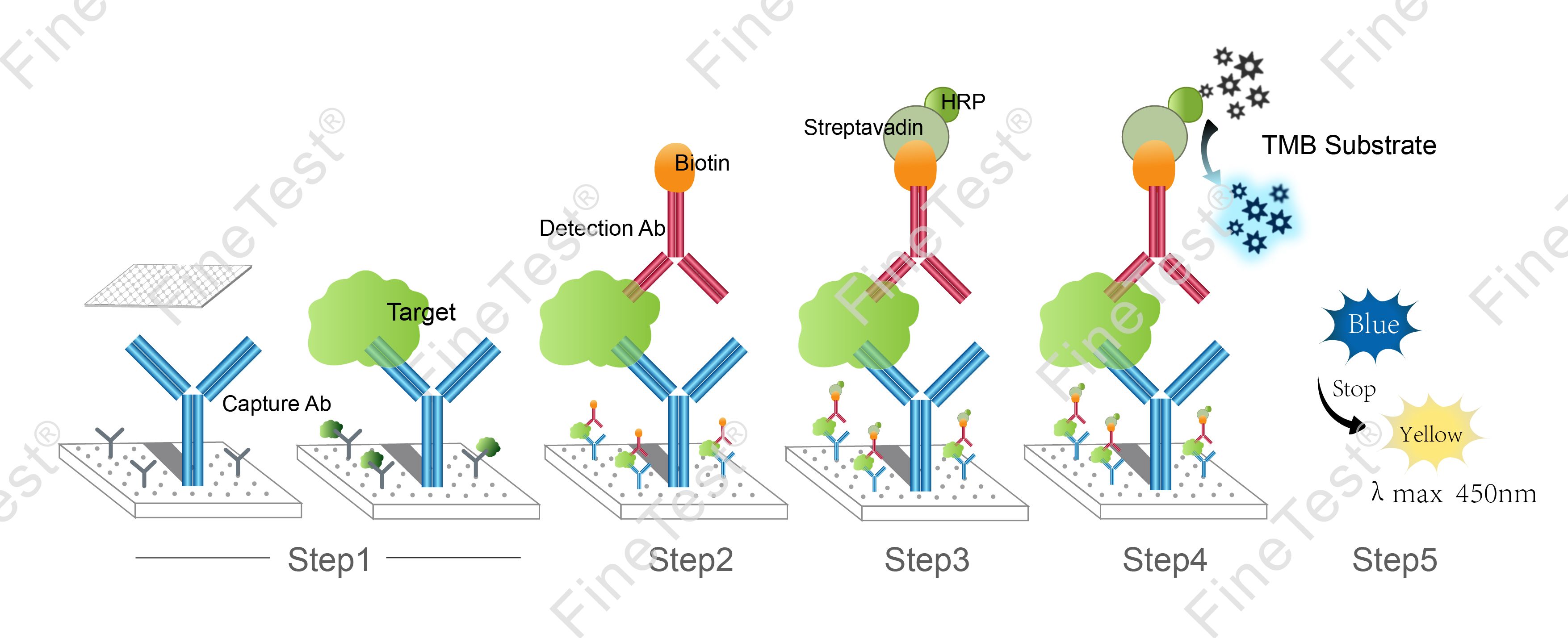
- Step 1: Add 100ul standard or sample into each well, seal the plate and statically incubate for 90 minutes at 37°C.
- Washing: Wash the plate twice without immersing.
- Step 2: Add 100ul biotin-antibody working solution, seal the plate and statically incubate for 60 minutes at 37°C.
- Washing: Wash the plate three times and immerse for 1min each time.
- Step 3: Add 100ul HRP-Streptavidin Conjugate (SABC) working solution, seal the plate and statically incubate for 30 minutes at 37°C.
- Washing: Wash the plate five times and immerse for 1min each time.
- Step 4: Add 90ul TMB substrate solution, seal the plate and statically incubate for 10-20 minutes at 37°C. (Accurate TMB visualization control is required.)
- Step 5: Add 50ul stop solution. Read at 450nm immediately and calculate.
- Standard Curve
-
This product is detected by QC department and meets performance required in the manual. (Laboratory Humidity: 20%-60%; Temperature: 18°C -25°C; Equilibrate TMB substrate to 37°C before staining. After adding into the ELISA wells, incubate for 15min at 37°C in dark.)
Due to different assay environments and operations, assay data below and standard curve are provided for reference. Experimenters should establish standard curve according to their own assay.
STD.(pg/ml) OD-1 OD-2 Average 0 0.091 0.096 0.093 3.9 0.194 0.204 0.198 7.81 0.263 0.276 0.268 15.6 0.344 0.362 0.351 31.25 0.495 0.52 0.505 62.5 0.814 0.855 0.831 125 1.273 1.338 1.299 250 2.195 2.306 2.239 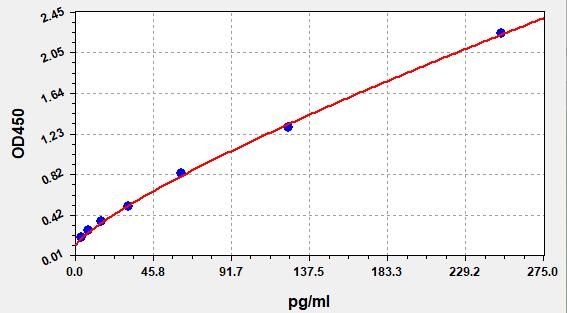
- Recovery
-
Add a certain amount of TNF-α into the sample. Calculate the recovery by comparing the measured value with the expected amount of TNF-α in the sample.
Sample Type Recovery Range(%) Average(%) serum(n=10) 95-105 98 EDTA plasma(n=10) 86-101 92 Heparin plasma(n=10) 92-103 97 - Linearity
-
Dilute the sample with a certain amount of TNF-α at 1:2, 1:4 and 1:8 to get the recovery range.
Sample Type 1:2 1:4 1:8 serum(n=10) 87-93% 85-105% 88-105% EDTA plasma(n=10) 85-101% 82-98% 85-94% Heparin plasma(n=10) 80-93% 82-100% 84-98% - Precision(%)
-
Intra-assay Precision: samples with low, medium and high concentration are tested 20 times on the same plate.
Inter-assay Precision: samples with low, medium and high concentration are tested 20 times on three different plates.
Item Intra-assay Precision Inter-assay Precision Sample 1 2 3 1 2 3 n 20 20 20 20 20 20 Mean (pg/ml) 7.3 30.03 123.93 8 30.65 127.4 Standard deviation 0.47 1.32 5.49 0.36 1.43 6.05 CV(%) 6.43 4.38 4.43 4.46 4.68 4.75 - Stability
-
Perform the stability test for the sealed kit at 37°C and 2-8°C and get relevant data.
ELISA kit(n=5) 37°C for 1 month 2-8°C for 6 months Average(%) 80 95-100
- Journal:
- Materials Today
- Author:
- State Key Laboratory of Bioactive Substance and Function of Natural Medicines, Department of Pharmaceutics, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100050, China
- Cited Date:
- 2023-08-11
- Product:
- Journal:
- Advanced Science
- Author:
- The People's Hospital of Gaozhou Guangdong Medical University Maoming 525200 China
- Cited Date:
- 2023-04-28
- Product:
- Journal:
- Advanced Science
- Author:
- Gaozhou People's Hospital, Maoming, 525200, China.
- Sample:
- supernatants
- Cited Date:
- 2024-03-29
- Product:
- Journal:
- Journal of Pharmaceutical Analysis
- Cited Date:
- 2022-08-11
- Product:
- Journal:
- Carbohydrate Polymers
- Cited Date:
- 2023-04-21
- Product:
- Journal:
- Cell Death & Differentiation
- Cited Date:
- 2021-03-04
- Product:
- Journal:
- Acta Biomaterialia
- Author:
- School of Medicine, South China University of Technology, Guangzhou 510006, PR China
- Cited Date:
- 2024-02-18
- Product:
- Journal:
- Frontiers in Immunology
- Author:
- Department of Gastroenterology, The First Affiliated Hospital, Jinan University, Guangzhou, Guangdong, China
- Cited Date:
- 2023-04-28
- Product:
- Journal:
- International Journal of Biological Macromolecules
- Author:
- College of Food Science and Engineering, Shanxi Agricultural University, Taigu, Shanxi 030801, China; Shanxi Key Laboratory of Edible Fungi for Loess Plateau, Taigu, Shanxi 030801, China.
- Cited Date:
- 2024-04-07
- Product:
- Journal:
- International Journal of Nanomedicine
- Author:
- Emergency Department, State Key Laboratory of Complex Severe and Rare Diseases, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, People’s Republic of China
- Cited Date:
- 2024-01-12
- Product:
- Journal:
- Journal of Medicinal Chemistry
- Author:
- Department of Pharmaceutical Chemistry, Faculty of Pharmacy, Horus University-Egypt, New Damietta 34518, Egypt
- Cited Date:
- 2024-01-05
- Product:
- Journal:
- Cell Death Discovery
- Cited Date:
- 2023-01-29
- Product:
- Journal:
- European Journal of Medicinal Chemistry
- Author:
- Jiangsu Key Laboratory of Drug Design and Optimization, Department of Medicinal Chemistry, School of Pharmacy, China Pharmaceutical University, Nanjing, 210009, China
- Cited Date:
- 2023-04-28
- Product:
- Journal:
- Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease
- Author:
- School of Pharmaceutical Sciences, Guangzhou University of Chinese Medicine, Guangzhou 510006, China
- Cited Date:
- 2024-02-18
- Product:
- Journal:
- Neurobiology of Disease
- Author:
- Laboratory of Immunoendocrinology, Department of Experimental Neuroendocrinology, Maj Institute of Pharmacology, Polish Academy of Sciences, 12 Sm?tna St., 31-343 Kraków, Poland
- Cited Date:
- 2023-07-14
- Product:
- Journal:
- Frontiers in Bioengineering and Biotechnology
- Cited Date:
- 2022-09-23
- Product:
- Journal:
- Aging
- Author:
- Department of ICU, The First People's Hospital of Linping District, Hangzhou 311100, China
- Cited Date:
- 2024-01-05
- Product:
- Journal:
- Journal of Agricultural and Food Chemistry
- Cited Date:
- 2022-12-15
- Product:
- Journal:
- Virulence
- Cited Date:
- 2021-11-11
- Product:
-
- Mouse TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Mouse IL-6(Interleukin 6) ELISA Kit
- Mouse IL-10(Interleukin-10) ELISA Kit
- Mouse IL-4(Interleukin 4) ELISA Kit
- Mouse IL-12(Interleukin 12) ELISA Kit
- Mouse CXCL1/GROα(Growth Regulated Oncogene Alpha) ELISA Kit
- Mouse IL-17α(Interleukin 17 alpha) ELISA Kit
- Mouse IL-2(Interleukin 2) ELISA Kit
- Mouse GROβ(Growth Regulated Oncogene Beta) ELISA Kit
- Mouse IL-21(Interleukin 21) ELISA Kit
- Mouse IL-33(Interleukin 33) ELISA Kit
- Journal:
- The FASEB Journal
- Cited Date:
- 2023-04-14
- Product:
- Journal:
- Cell Proliferation
- Cited Date:
- 2020-09-01
- Product:
-
- Mouse TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Mouse IL-6(Interleukin 6) ELISA Kit
- Mouse ALT(Alanine Transaminase) ELISA Kit
- Mouse AST(Aspartate Aminotransferase) ELISA Kit
- Mouse NOS2/iNOS(Nitric Oxide Synthase 2, Inducible) ELISA Kit
- Mouse CTXI(Cross Linked C-telopeptide of Type I Collagen) ELISA Kit
- Mouse OC/BGP(Osteocalcin) ELISA Kit
- Mouse BALP(Bone Alkaline Phosphatase) ELISA Kit
- Mouse Gsr(Glutathione reductase, mitochondrial) ELISA Kit
- Mouse Txn(Thioredoxin) ELISA Kit
- Journal:
- International Journal of Molecular Sciences
- Author:
- Department of Cosmeceutics, China Medical University, Taichung 406, Taiwan
- Cited Date:
- 2023-08-11
- Product:
- Journal:
- Laboratory Investigation
- Cited Date:
- 2023-01-29
- Product:
- Journal:
- Food Bioscience
- Cited Date:
- 2023-01-28
- Product:
- Journal:
- Bioorganic Chemistry
- Cited Date:
- 2022-03-18
- Product:
- Journal:
- Journal of Ethnopharmacology
- Cited Date:
- 2022-12-22
- Product:
- Journal:
- Chemico-Biological Interactions
- Author:
- School of Biosciences, Faculty of Health and Medical Sciences Taylor's University Lakeside Campus, No1 Jalan Taylor's, 47500, Subang Jaya, Malaysia
- Cited Date:
- 2023-04-28
- Product:
- Journal:
- European Journal of Pharmacology
- Author:
- Department of Pharmacology, Xiangya School of Pharmaceutical Sciences, Central South University, Changsha, 410078, China
- Cited Date:
- 2023-08-11
- Product:
- Journal:
- ACS Chemical Neuroscience
- Author:
- Department of Biosciences and Biomedical Engineering, Indian Institute of Technology Indore, Indore, Madhya Pradesh 453552, India.
- Cited Date:
- 2024-03-15
- Product:
- Journal:
- Bioorganic Chemistry
- Cited Date:
- 2021-07-02
- Product:
- Journal:
- Scientific Reports
- Author:
- Health Sciences Faculty, UNOESTE (University of Western Sao Paulo), 700, Jose Bongiovani St., Cidade Universitária, Presidente Prudente, Sao Paulo, 19050-920, Brazil
- Cited Date:
- 2023-11-03
- Product:
- Journal:
- Scientific Reports
- Author:
- Department of Paramedical and Allied Health Sciences, Midnapore City College, Midnapore, 721129, West Bengal, India
- Cited Date:
- 2023-09-15
- Product:
- Journal:
- European Journal of Pharmacology
- Cited Date:
- 2022-01-13
- Product:
- Journal:
- Neurochemical Research
- Author:
- Department of Anesthesiology, Affiliated Hospital of North Sichuan Medical College, Nanchong, China
- Cited Date:
- 2023-09-01
- Product:
- Journal:
- Nutritional Neuroscience
- Cited Date:
- 2023-04-07
- Product:
- Journal:
- RSC Advances
- Author:
- Department of Pharmacognosy, Faculty of Pharmacy, Kafrelsheikh University, P.O. Box 33516, Kafrelsheikh, Egypt
- Cited Date:
- 2023-09-08
- Product:
- Journal:
- Prostaglandins & Other Lipid Mediators
- Author:
- Yeditepe University, 26 August Settlement, Atasehir, Istanbul 34755, Turkey
- Cited Date:
- 2023-05-05
- Product:
- Journal:
- Heliyon
- Cited Date:
- 2023-04-07
- Product:
- Journal:
- Biomolecules and Biomedicine
- Cited Date:
- 2023-02-16
- Product:
- Journal:
- Immunopharmacology and Immunotoxicology
- Author:
- Department of Respiratory Medicine, Baoshan Branch of Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
- Cited Date:
- 2023-07-14
- Product:
- Journal:
- NeuroMolecular Medicine
- Author:
- Department of Burn and Plastic Surgery, Shenzhen Longhua District Central Hospital, Shenzhen, 518110, Guangdong, China
- Cited Date:
- 2023-10-13
- Product:
- Journal:
- ??Pancreas
- Cited Date:
- 2021-11-12
- Product:
- Journal:
- BioMed Research International
- Cited Date:
- 2022-09-01
- Product:
- Journal:
- Journal of Molecular Histology
- Author:
- Department of Burn and Plastic Surgery, Shenzhen Longhua District Central Hospital, Shenzhen, 518110, Guangdong, China
- Cited Date:
- 2023-09-15
- Product:
- Journal:
- Autoimmunity
- Cited Date:
- 2023-03-24
- Product:
-
- Mouse TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Mouse IL-6(Interleukin 6) ELISA Kit
- Mouse IL-10(Interleukin-10) ELISA Kit
- Mouse IL-4(Interleukin 4) ELISA Kit
- Mouse TGF-β1(Transforming Growth Factor β1) ELISA Kit
- Mouse IFN-γ(Interferon Gamma) ELISA Kit
- Mouse His(Histamine) ELISA Kit
- Mouse OVA sIgG1(Ovalbumin Specific IgG1) ELISA Kit
- Mouse IL-17A/F(Interleukin 17A/F)ELISA Kit
- Mouse IL-5(Interleukin 5) ELISA Kit
- Mouse OVA sIgG2a(Ovalbumin Specific IgG2a) ELISA Kit
- Mouse OVA sIgE(Ovalbumin Specific IgE) ELISA Kit
- Mouse Foxp3(Forkhead box protein P3) ELISA Kit
- Journal:
- Clinics
- Author:
- Department of Orthopedics, Jianhu People's Hospital, Yancheng, Jiangsu, China
- Cited Date:
- 2023-07-28
- Product:
- Journal:
- Chemical and Pharmaceutical Bulletin
- Cited Date:
- 2023-04-07
- Product:
- Journal:
- Journal of Food Biochemistry
- Cited Date:
- 2020-09-03
- Product:
- Journal:
- Scientia Pharmaceutica
- Cited Date:
- 2020-08-10
- Product:
- Journal:
- Annual Research & Review in Biology
- Cited Date:
- 2021-04-02
- Product:
- Journal:
- Research Square
- Author:
- Nanchang University
- Cited Date:
- 2024-02-02
- Product:
- Journal:
- Research Square
- Author:
- Ahmadu Bello University
- Cited Date:
- 2024-02-02
- Product:
- Journal:
- SSRN
- Author:
- Department of Anesthesiology, the Affiliated Hospital of North Sichuan Medical College, Nanchong, China
- Cited Date:
- 2023-11-03
- Product:
- Journal:
- Indonesian Journal of Pharmacy
- Cited Date:
- 2022-01-06
- Product:
- Journal:
- Egyptian Journal of Chemistry
- Cited Date:
- 2022-01-07
- Product:
- Journal:
- Pharmacognosy Journal
- Cited Date:
- 2021-01-21
- Product:
- Journal:
- Medicinski Glasnik
- Cited Date:
- 2020-05-04
- Product:
- Journal:
- Journal of Natural Science, Biology and Medicine
- Cited Date:
- 2021-02-20
- Product: