FineTest ELISA kit contributes to the research on SARS-CoV-2 Omicron. The immunoassay is designed to measure S-RBD IgG level concentration in serum.
Publication Details
Article Title: Antibody responses to SARS-CoV-2 Omicron infection in patients with hematological malignancies: A multicenter, prospective cohort study
Journal Title: Journal of Medical Virology
DOI: 10.1002/jmv.29300
IF: 12.7
PMID: 38063070
Abstract: Little is known about antibody responses to natural Omicron infection and the risk factors for poor responders in patients with hematological malignancies (HM). We conducted a multicenter, prospective cohort study during the latest Omicron wave in Chongqing, China, aiming to compare the antibody responses, as assessed by IgG levels of anti-receptor binding domain of spike protein (anti-S-RBD), to Omicron infection in the HM cohort (HMC) with healthy control cohort (HCC), and solid cancer cohort (SCC). In addition, we intend to explore the risk factors for poor responders in the HMC. Among the 466 HM patients in this cohort, the seroconversion rate was 92.7%, no statistically difference compared with HCC (98.2%, p = 0.0513) or SCC (100%, p = 0.1363). The median anti-S-RBD IgG titer was 29.9 ng/mL, significantly lower than that of HCC (46.9 ng/mL, p < 0.0001) or SCC (46.2 ng/mL, p < 0.0001). Risk factors associated with nonseroconversion included no COVID-19 vaccination history (odds ratio [OR] = 4.58, 95% confidence interval [CI]: 1.75–12.00, p = 0.002), clinical course of COVID-19 ≤ 7 days (OR = 2.86, 95% CI: 1.31–6.25, p = 0.008) and severe B-cell reduction (0–10/μL) (OR = 3.22, 95% CI: 1.32–7.88, p = 0.010). Risk factors associated with low anti-S-RBD IgG titer were clinical course of COVID-19 ≤ 7 days (OR = 2.58, 95% CI: 1.59–4.18, p < 0.001) and severe B-cell reduction (0–10/μL) (OR = 2.87, 95% CI: 1.57–5.24, p < 0.001). This study reveals a poor antibody responses to Omicron (BA.5.2.48) infection in HM patients and identified risk factors for poor responders. Highlights that HM patients, especially those with these risk factors, may be susceptible to SARS-CoV-2 reinfection, and the postinfection vaccination strategies for these patients should be tailored.
Keywords: COVID-19, Omicron, SARS-CoV-2, antibody responses, Hematological Malignancies
Immunoassay
| FineTest Product | Sample | Species | Detection Target |
| Human anti-SARS-CoV2(S-RBD) (Omicron,B.1.1.529) IgG ELISA Kit(EH4971) | serum | Human | S-RBD IgG |
Validated Image
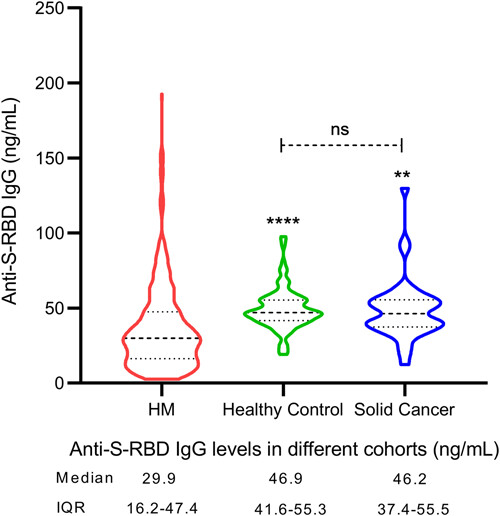
Figure Source: J Med Virol. 2023 Dec;95(12):e29300. doi: 10.1002/jmv.29300.
