FineTest Elisa kit contributes to the research on liver cancer chemotherapy. The immunoassay is designed to measure BTC and CCL28 concentration in serum.
Publication Details
Article Title: Lenvatinib, toripalimab plus hepatic arterial infusion chemotherapy in patients with high-risk advanced hepatocellular carcinoma: A biomolecular exploratory, phase II trial
Journal Title: European Journal of Cancer
DOI: 10.1016/j.ejca.2022.07.005
IF: 10.002
PMID: 35981413
Abstract: Introduction: The combination of lenvatinib, toripalimab and hepatic arterial infusion chemotherapy (HAIC) with oxaliplatin, leucovorin, and 5-fluorouracil (FOLFOX) suggested encouraging antitumour activity in our retrospective study. We hereby prospectively establish the efficacy, safety and predictive biomarkers of the combination therapy as a first-line treatment in patients with high-risk advanced hepatocellular carcinoma (HCC). Materials and methods: This phase II, single-centre, single-arm trial enrolled advanced HCC participants with high-risk. Of 51 screened participants, 36 received lenvatinib, toripalimab plus FOLFOX-HAIC. Participants received 21-day treatment cycles of lenvatinib, toripalimab, and FOLFOX-HAIC. The primary end-point was the progression-free survival (PFS) rate per RECIST at six months. Results: Thirty-six participants (86.1% with high-risk features) were enrolled in our study. The primary end-point was met with a PFS rate of 80.6% (95% CI, 64.0%-91.8%) at six months. The median PFS was 10.4 months (95% CI, 5.8-15.0), and the median OS was not reached at the prespecified final analysis and was 17.9 months (95% CI, 14.5-21.3) after follow-up was extended. The ORR per RECIST was 63.9%, and per mRECIST was 66.7%. The median duration of response was 14.4 months (95% CI, 8.9-19.9). The most common adverse events were thrombocytopenia, elevated aspartate aminotransferase, and hypertension, and no treatment-related death was reported. Participants with low levels of both CCL28 and BTC had unsatisfactory prognosis. Conclusions: Lenvatinib, toripalimab and FOLFOX-HAIC showed safe and encouraging antitumour activity for advanced HCC with high-risk features. The levels of CCL28 and BTC might be the predictive biomarkers for the triple combination therapy.
Keywords: Hepatic arterial infusion chemotherapy; High-risk advanced hepatocellular carcinoma; Lenvatinib; Predictive biomarkers; Toripalimab
Immunoassay
| FineTest Product | Sample | Species | Detection Target |
| Human BTC(Betacellulin) ELISA Kit (EH0044) | serum | human | BTC |
| Human CCL28(C-C motif chemokine 28) ELISA Kit (EH0675) | CCL28 |
Validated Image
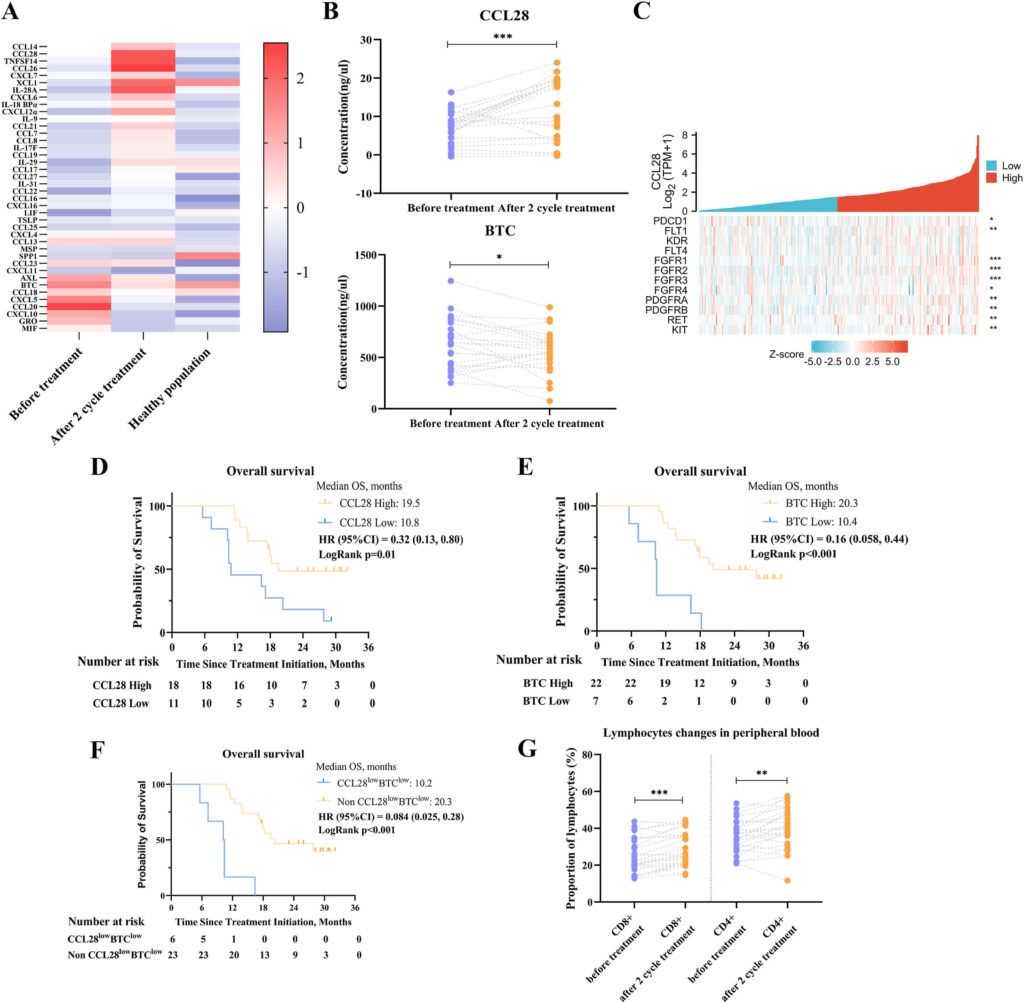
Figure Source: Eur J Cancer. 2022 Aug 15;174:68-77. doi: 10.1016/j.ejca.2022.07.005.
Fig. 4. The levels of CCL28 or BTC before the treatment might be a predictive biomarker for the efficacy of combination therapy. (A) The heatmap of quantitative measurement of human cytokines in the pre-experiment. (B) The change of peripheral blood level of CCL28 (upper) and BTC (lower) before and after the treatment. (C) The expression of PD-1 and lenvatinib target between CCL28 high group and CCL28 low group from TCGA database. (D) Kaplan–Meier curves of overall survival between CCL28 high group and CCL28 low group (cut-off value, 5.9). (E) Kaplan–Meier curves of overall survival between BTC high group and BTC low group (cut-off value, 387.8). (F) Kaplan–Meier curves of overall survival between CCL28lowBTClow group and non-CCL28lowBTClow group. (G) The number of CD8+ (left) and CD4+ (right) T cells before and after the treatment.
