Products
Human TGF-β1(Transforming Growth Factor Beta 1) ELISA Kit
This kit is based on Double antibody-Sandwich ELISA detection method and takes 4h assay time. The microplate provided in this kit has been precoated with anti TGF-β1 antibody. Add standard and properly diluted sample into relevant well respectively. After incubation, wash unbound components. Add biotinylated detection antibody. Then, it binds with TGF-β1 bound to precoated antibody. Wash unbound components and add HRP-Streptavidin Conjugate (SABC). Wash unbound components again and add TMB substrate solution. Then, TMB was catalyzed by HRP to produce a blue color product that turned yellow after adding a stop solution. Read the O.D. absorbance at 450nm in a microplate reader. Calculate the concentration of TGF-β1 in the sample by plotting standard curve. The concentration of the target substance is proportional to the OD450 value.
- Catalogue No.:
- EH0287
- Alias:
- TGF-β1 ELISA Kit, Transforming Growth Factor Beta 1 ELISA Kit, TGF-B1 ELISA Kit, TGFB ELISA Kit, TGFbeta ELISA Kit, CED ELISA Kit, DPD1 ELISA Kit, TGFβ1 ELISA Kit
- Species:
- Human
- Range:
- 31.25-2000pg/ml
- Sensitivity:
- 18.75pg/ml
- SPECIFICATIONS
- CITATIONS
- Product Name
- Human TGF-β1(Transforming Growth Factor Beta 1) ELISA Kit
- Alias
- TGF-β1 ELISA Kit, Transforming Growth Factor Beta 1 ELISA Kit, TGF-B1 ELISA Kit, TGFB ELISA Kit, TGFbeta ELISA Kit, CED ELISA Kit, DPD1 ELISA Kit, TGFβ1 ELISA Kit
- Catalogue No.
- EH0287
- Size
- 48T/96T
- Species
- Human
- UniProt No.
- P01137
- Sample Type
- Serum, Plasma, Cell Culture Supernatant, cell or tissue lysate, Other liquid samples
- Detection Method
- Sandwich ELISA, Double Antibody
- Detection Wavelength
- OD450
- Reaction Duration
- 4 hours
- Range
- 31.25-2000pg/ml
- Sensitivity
- 18.75pg/ml
- Storage
- 2-8°C(Sealed), Don't cryopreserve.
- Specificity
- Specifically binds with TGF-β1 , no obvious cross reaction with other analogues.
- ELISA Kit Components
Kit Components Item Size(48T) Size(96T) Storage Condition for Opened Kit E001 ELISA Microplate(Dismountable) 8×6 8×12 Put the rest strips into a sealed foil bag with the desiccant. Stored for 1 month at 2-8°C; Stored for 6 month at -20°C E002 Lyophilized Standard 1vial 2vial Put the rest standards into a desiccant bag. Stored for 1 month at 2-8°C; Stored for 6 month at -20°C E003 Biotin-labeled Antibody(Concentrated, 100X) 60ul 120ul 2-8°C (Avoid Direct Light) E034 HRP-Streptavidin Conjugate(SABC, 100X) 60ul 120ul E024 TMB Substrate 5ml 10ml E039 Sample Dilution Buffer 10ml 20ml 2-8°C E040 Antibody Dilution Buffer 5ml 10ml E049 SABC Dilution Buffer 5ml 10ml E026 Stop Solution 5ml 10ml E038 Wash Buffer(Concentrated, 25X) 15ml 30ml E008 Preprocess Agent A 1vial 1.5ml 2vial 1.5ml E009 Preprocess Agent B 1vial 1.5ml 2vial 1.5ml E006 Plate Sealer 3 pieces 5 pieces E007 Product Description 1 copy 1 copy - Required Instruments and Reagents
-
- Microplate reader (wavelength: 450nm)
- 37°C incubator (CO2 incubator for cell culture is not recommenced.)
- Automated plate washer or multi-channel pipette/5ml pipettor (for manual washing purpose)
- Precision single (0.5-10μL, 5-50μL, 20-200μL, 200-1000μL) and multi-channel pipette with disposable tips(Calibration is required before use.)
- Sterile tubes and Eppendorf tubes with disposable tips
- Absorbent paper and loading slot
- Deionized or distilled water
- Assay Procedure Summary
-
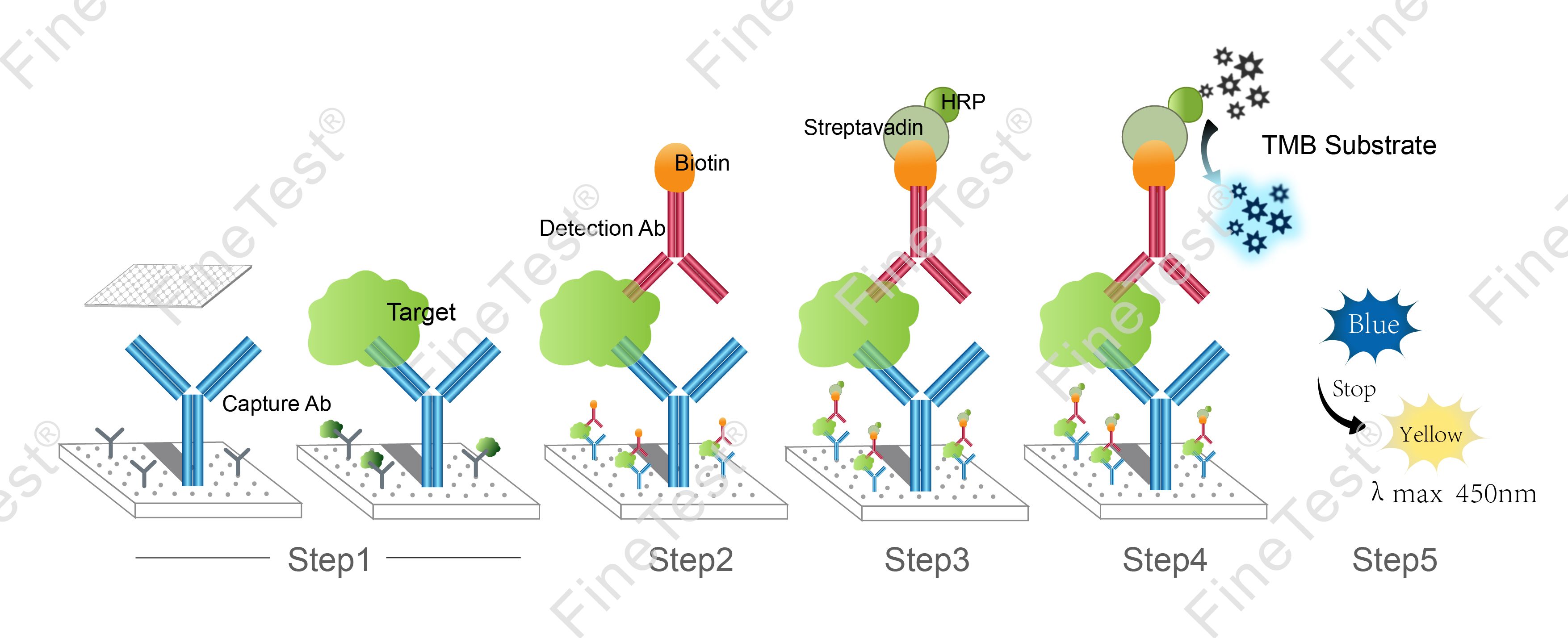
- Step 1: Add 100ul standard or sample into each well, seal the plate and statically incubate for 90 minutes at 37°C.
- Washing: Wash the plate twice without immersing.
- Step 2: Add 100ul biotin-antibody working solution, seal the plate and statically incubate for 60 minutes at 37°C.
- Washing: Wash the plate three times and immerse for 1min each time.
- Step 3: Add 100ul HRP-Streptavidin Conjugate (SABC) working solution, seal the plate and statically incubate for 30 minutes at 37°C.
- Washing: Wash the plate five times and immerse for 1min each time.
- Step 4: Add 90ul TMB substrate solution, seal the plate and statically incubate for 10-20 minutes at 37°C. (Accurate TMB visualization control is required.)
- Step 5: Add 50ul stop solution. Read at 450nm immediately and calculate.
- Standard Curve
-
This product is detected by QC department and meets performance required in the manual. (Laboratory Humidity: 20%-60%; Temperature: 18°C -25°C; Equilibrate TMB substrate to 37°C before staining. After adding into the ELISA wells, incubate for 15min at 37°C in dark.)
Due to different assay environments and operations, assay data below and standard curve are provided for reference. Experimenters should establish standard curve according to their own assay.
STD.(pg/ml) OD-1 OD-2 Average 0 0.127 0.133 0.129 31.25 0.251 0.264 0.256 62.5 0.427 0.449 0.436 125 0.646 0.679 0.659 250 1.023 1.075 1.044 500 1.427 1.5 1.457 1000 1.834 1.928 1.871 2000 2.098 2.205 2.141 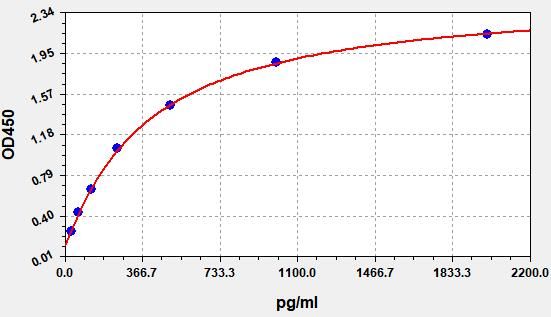
- Recovery
-
Add a certain amount of TGF-β1 into the sample. Calculate the recovery by comparing the measured value with the expected amount of TGF-β1 in the sample.
Sample Type Recovery Range(%) Average(%) serum(n=10) 85-103 94 EDTA plasma(n=10) 88-104 97 Heparin plasma(n=10) 88-105 94 - Linearity
-
Dilute the sample with a certain amount of TGF-β1 at 1:2, 1:4 and 1:8 to get the recovery range.
Sample Type 1:2 1:4 1:8 serum(n=10) 90-103% 82-99% 83-100% EDTA plasma(n=10) 90-101% 85-101% 82-89% Heparin plasma(n=10) 88-94% 82-101% 80-96% - Precision(%)
-
Intra-assay Precision: samples with low, medium and high concentration are tested 20 times on the same plate.
Inter-assay Precision: samples with low, medium and high concentration are tested 20 times on three different plates.
Item Intra-assay Precision Inter-assay Precision Sample 1 2 3 1 2 3 n 20 20 20 20 20 20 Mean (pg/ml) 59.21 241.8 971.1 59.69 250.5 984.2 Standard deviation 2.72 13.42 54.38 3.36 14.65 58.26 CV(%) 4.59 5.55 5.6 5.63 5.85 5.92 - Stability
-
Perform the stability test for the sealed kit at 37°C and 2-8°C and get relevant data.
ELISA kit(n=5) 37°C for 1 month 2-8°C for 6 months Average(%) 80 95-100
- Journal:
- Molecular Cancer
- Author:
- Digestive Diseases Center, Guangdong Provincial Key Laboratory of Digestive Cancer Research, The Seventh Affiliated Hospital, Sun Yat-Sen University, Shenzhen, China.
- Sample:
- cell culture
- Cited Date:
- 2024-03-15
- Product:
- Journal:
- Journal of Experimental & Clinical Cancer Research
- Author:
- Preclinical Models and New Therapeutic Agents Unit, IRCCS Regina Elena National Cancer Institute, Rome, Italy
- Cited Date:
- 2023-07-28
- Product:
- Journal:
- Oncogene
- Cited Date:
- 2022-12-02
- Product:
- Journal:
- Frontiers in Immunology
- Cited Date:
- 2022-03-18
- Product:
- Journal:
- Journal of Crohn's and Colitis
- Cited Date:
- 2019-01-25
- Product:
- Journal:
- Cancer Immunology, Immunotherapy
- Cited Date:
- 2022-09-15
- Product:
- Journal:
- International Journal of Molecular Sciences
- Cited Date:
- 2022-10-27
- Product:
- Journal:
- International Journal of Molecular Sciences
- Cited Date:
- 2022-07-29
- Product:
-
- Human IL-6(Interleukin 6) ELISA Kit
- Human TGF-β1(Transforming Growth Factor Beta 1) ELISA Kit
- Human IL-10(Interleukin 10) ELISA Kit
- Human IL-17A(Interleukin 17 A) ELISA Kit
- Human IFN-γ(Interferon gamma) ELISA Kit
- Human IL-35 (Interleukin 35) ELISA Kit
- Human IL-23(Interleukin 23) ELISA Kit
- Human IL-22(Interleukin-22) ELISA Kit
- Human IL-21(Interleukin-21) ELISA Kit
- Human IL-17 F(Interleukin 17F) ELISA Kit
- Journal:
- Journal of Clinical Medicine
- Cited Date:
- 2020-04-12
- Product:
-
- Human TGF-β1(Transforming Growth Factor Beta 1) ELISA Kit
- Human VEGF(Vascular Endothelial Cell Growth Factor) ELISA Kit
- Human FGF2(Heparin-binding growth factor 2) ELISA Kit
- Human PDGF-BB(Platelet Derived Growth Factor-BB) ELISA Kit
- Human BMP-2(Bone Morphogenetic Protein 2) ELISA Kit
- Human PDGF-AB(Platelet-Derived Growth Factor AB) ELISA Kit
- Journal:
- Nanomaterials
- Author:
- Department of Biotechnology and Life Sciences, University of Insubria, 21100 Varese, Italy
- Cited Date:
- 2023-09-08
- Product:
- Journal:
- Therapeutic Advances in Musculoskeletal Disease
- Author:
- Department of Experimental Medicine, University of Campania ‘Luigi Vanvitelli’, Naples, Italy
- Cited Date:
- 2024-01-12
- Product:
- Journal:
- Journal of Biochemical and Molecular Toxicology
- Author:
- Department of Medical Biochemistry, Unit of Biochemistry and Molecular Biology, Faculty of Medicine Cairo University Cairo Egypt
- Cited Date:
- 2023-10-27
- Product:
- Journal:
- Clinical Oral Investigations
- Author:
- Department of Periodontology, Faculty of Dentistry, Cukurova University, Adana, Turkey
- Cited Date:
- 2024-01-26
- Product:
- Journal:
- Beni-Suef University Journal of Basic and Applied Sciences
- Author:
- Biotechnology School, Nile University, Giza, Egypt
- Cited Date:
- 2023-07-28
- Product:
- Journal:
- Journal of Endodontics
- Cited Date:
- 2019-12-04
- Product:
- Journal:
- Immunity, Inflammation and Disease
- Cited Date:
- 2022-11-04
- Product:
- Journal:
- Journal of Clinical Pathology
- Cited Date:
- 2021-05-06
- Product:
- Journal:
- Dermatologic Therapy
- Cited Date:
- 2020-08-07
- Product:
- Journal:
- Open Life Sciences
- Author:
- School of Medicine, Shaoxing University, Shaoxing 312000, Zhejiang, China
- Cited Date:
- 2023-10-08
- Product:
- Journal:
- Turkish Journal of Biology
- Author:
- Ankara University Cancer Research Institute, Ankara, Turkiye
- Cited Date:
- 2023-09-22
- Product:
- Journal:
- Journal of Orthopaedic Surgery and Research
- Cited Date:
- 2020-11-19
- Product:
- Journal:
- Antarctic Science
- Cited Date:
- 2021-09-24
- Product:
- Journal:
- Medicinski Glasnik
- Cited Date:
- 2020-06-15
- Product:
- Journal:
- F?rat University Medical Journal of Health Sciences
- Cited Date:
- 2022-04-07
- Product:
- Journal:
- Health and environmental issues
- Cited Date:
- 2019-08-15
- Product:
- Journal:
- The Medical Journal of Cairo University
- Cited Date:
- 2019-09-29
- Product:
- Journal:
- Dental Hypotheses
- Cited Date:
- 2018-07-13
- Product:
- Journal:
- Archives of Pharmaceutical Sciences Ain Shams University
- Cited Date:
- 2021-06-18
- Product:
- Journal:
- Diabetes Metab Syndr Obes
- Cited Date:
- 2018-10-26
- Product:
- Journal:
- Научный форум: Медицина, биология и химия
- Cited Date:
- 2021-01-26
- Product:
- Journal:
- Research Square
- Cited Date:
- 2020-05-20
- Product:
- Journal:
- Journal of Nature and Science of Medicine
- Cited Date:
- 2023-01-13
- Product:
- Journal:
- Journal of Oral Medicine and Dental Research
- Author:
- Orthodontics, Pedodontics and Prevention Department Faculty of Dentistry, Sana'a University, Republic of Yemen
- Cited Date:
- 2023-09-28
- Product: