Products
Rat IL-6(Interleukin-6) ELISA Kit
This kit is based on Double antibody-Sandwich ELISA detection method and takes 4h assay time. The microplate provided in this kit has been precoated with anti IL-6 antibody. Add standard and properly diluted sample into relevant well respectively. After incubation, wash unbound components. Add biotinylated detection antibody. Then, it binds with IL-6 bound to precoated antibody. Wash unbound components and add HRP-Streptavidin Conjugate (SABC). Wash unbound components again and add TMB substrate solution. Then, TMB was catalyzed by HRP to produce a blue color product that turned yellow after adding a stop solution. Read the O.D. absorbance at 450nm in a microplate reader. Calculate the concentration of IL-6 in the sample by plotting standard curve. The concentration of the target substance is proportional to the OD450 value.
- Catalogue No.:
- ER0042
- Alias:
- IL-6 ELISA Kit, Interleukin 6 ELISA Kit, IL6 ELISA Kit, BSF2 ELISA Kit, HGF ELISA Kit, HSF ELISA Kit, IFNB2 ELISA Kit, CDF ELISA Kit
- Species:
- Rat
- Range:
- 62.5-4000pg/ml
- Sensitivity:
- 37.5pg/ml
- SPECIFICATIONS
- CITATIONS
- Product Name
- Rat IL-6(Interleukin-6) ELISA Kit
- Alias
- IL-6 ELISA Kit, Interleukin 6 ELISA Kit, IL6 ELISA Kit, BSF2 ELISA Kit, HGF ELISA Kit, HSF ELISA Kit, IFNB2 ELISA Kit, CDF ELISA Kit
- Catalogue No.
- ER0042
- Size
- 48T/96T
- Species
- Rat
- UniProt No.
- P20607
- Sample Type
- Serum, Plasma, Cell Culture Supernatant, cell or tissue lysate, Other liquid samples
- Detection Method
- Sandwich ELISA, Double Antibody
- Detection Wavelength
- OD450
- Reaction Duration
- 4 hours
- Range
- 62.5-4000pg/ml
- Sensitivity
- 37.5pg/ml
- Storage
- 2-8°C(Sealed), Don't cryopreserve.
- Specificity
- Specifically binds with IL-6 , no obvious cross reaction with other analogues.
- ELISA Kit Components
Kit Components Item Size(48T) Size(96T) Storage Condition for Opened Kit E001 ELISA Microplate(Dismountable) 8×6 8×12 Put the rest strips into a sealed foil bag with the desiccant. Stored for 1 month at 2-8°C; Stored for 6 month at -20°C E002 Lyophilized Standard 1vial 2vial Put the rest standards into a desiccant bag. Stored for 1 month at 2-8°C; Stored for 6 month at -20°C E003 Biotin-labeled Antibody(Concentrated, 100X) 60ul 120ul 2-8°C (Avoid Direct Light) E034 HRP-Streptavidin Conjugate(SABC, 100X) 60ul 120ul E024 TMB Substrate 5ml 10ml E039 Sample Dilution Buffer 10ml 20ml 2-8°C E040 Antibody Dilution Buffer 5ml 10ml E049 SABC Dilution Buffer 5ml 10ml E026 Stop Solution 5ml 10ml E038 Wash Buffer(Concentrated, 25X) 15ml 30ml E006 Plate Sealer 3 pieces 5 pieces E007 Product Description 1 copy 1 copy - Required Instruments and Reagents
-
- Microplate reader (wavelength: 450nm)
- 37°C incubator (CO2 incubator for cell culture is not recommenced.)
- Automated plate washer or multi-channel pipette/5ml pipettor (for manual washing purpose)
- Precision single (0.5-10μL, 5-50μL, 20-200μL, 200-1000μL) and multi-channel pipette with disposable tips(Calibration is required before use.)
- Sterile tubes and Eppendorf tubes with disposable tips
- Absorbent paper and loading slot
- Deionized or distilled water
- Assay Procedure Summary
-
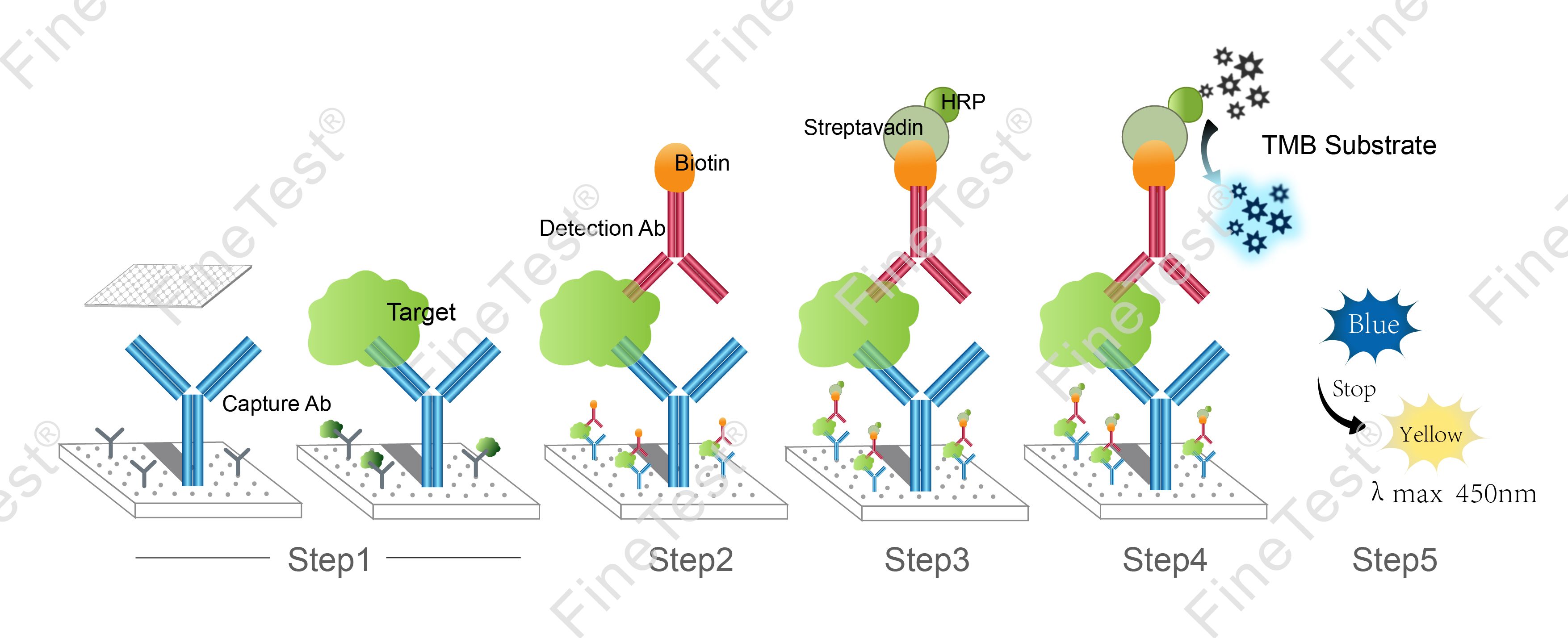
- Step 1: Add 100ul standard or sample into each well, seal the plate and statically incubate for 90 minutes at 37°C.
- Washing: Wash the plate twice without immersing.
- Step 2: Add 100ul biotin-antibody working solution, seal the plate and statically incubate for 60 minutes at 37°C.
- Washing: Wash the plate three times and immerse for 1min each time.
- Step 3: Add 100ul HRP-Streptavidin Conjugate (SABC) working solution, seal the plate and statically incubate for 30 minutes at 37°C.
- Washing: Wash the plate five times and immerse for 1min each time.
- Step 4: Add 90ul TMB substrate solution, seal the plate and statically incubate for 10-20 minutes at 37°C. (Accurate TMB visualization control is required.)
- Step 5: Add 50ul stop solution. Read at 450nm immediately and calculate.
- Standard Curve
-
This product is detected by QC department and meets performance required in the manual. (Laboratory Humidity: 20%-60%; Temperature: 18°C -25°C; Equilibrate TMB substrate to 37°C before staining. After adding into the ELISA wells, incubate for 15min at 37°C in dark.)
Due to different assay environments and operations, assay data below and standard curve are provided for reference. Experimenters should establish standard curve according to their own assay.
STD.(pg/ml) OD-1 OD-2 Average 0 0.129 0.136 0.132 62.5 0.168 0.176 0.171 125 0.179 0.188 0.183 250 0.218 0.229 0.222 500 0.319 0.335 0.325 1000 0.61 0.641 0.622 2000 1.157 1.216 1.181 4000 2.011 2.114 2.052 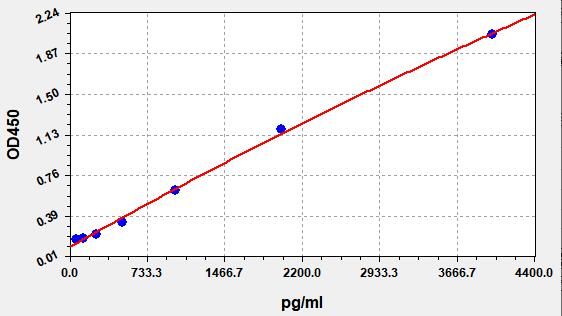
- Recovery
-
Add a certain amount of IL-6 into the sample. Calculate the recovery by comparing the measured value with the expected amount of IL-6 in the sample.
Sample Type Recovery Range(%) Average(%) serum(n=10) 87-105 98 EDTA plasma(n=10) 85-103 96 Heparin plasma(n=10) 94-105 100 - Linearity
-
Dilute the sample with a certain amount of IL-6 at 1:2, 1:4 and 1:8 to get the recovery range.
Sample Type 1:2 1:4 1:8 serum(n=10) 86-103% 86-99% 86-98% EDTA plasma(n=10) 92-98% 90-101% 83-100% Heparin plasma(n=10) 81-100% 80-99% 86-98% - Precision(%)
-
Intra-assay Precision: samples with low, medium and high concentration are tested 20 times on the same plate.
Inter-assay Precision: samples with low, medium and high concentration are tested 20 times on three different plates.
Item Intra-assay Precision Inter-assay Precision Sample 1 2 3 1 2 3 n 20 20 20 20 20 20 Mean (pg/ml) 121.8 501 1984 124.9 497.1 1995 Standard deviation 6.15 25.95 103.76 6.57 23.61 105.54 CV(%) 5.05 5.18 5.23 5.26 4.75 5.29 - Stability
-
Perform the stability test for the sealed kit at 37°C and 2-8°C and get relevant data.
ELISA kit(n=5) 37°C for 1 month 2-8°C for 6 months Average(%) 80 95-100
- Journal:
- Biomedicine & Pharmacotherapy
- Cited Date:
- 2022-03-11
- Product:
- Journal:
- DRUG INVENTION TODAY
- Cited Date:
- 2019-08-25
- Product:
- Journal:
- Journal of Neuroinflammation
- Cited Date:
- 2020-08-23
- Product:
- Journal:
- International Journal of Molecular Sciences
- Author:
- Key Laboratory of Shaanxi Province for Craniofacial Precision Medicine Research, College of Stomatology, Xi’an Jiaotong University, Xi’an 710004, China
- Cited Date:
- 2023-12-01
- Product:
- Journal:
- Journal of Ethnopharmacology
- Author:
- Regional Plant Resource Centre, Medicinal & Aromatic Plant Division, Forest & Environment Department, Govt. of Odisha, Nayapalli, Bhubaneswar, 751015, India
- Cited Date:
- 2023-07-28
- Product:
- Journal:
- Phytomedicine Plus
- Cited Date:
- 2021-09-02
- Product:
- Journal:
- Frontiers in Microbiology
- Author:
- Periodontics Department, College of Dentistry, Hawler Medical University, Erbil, Iraq
- Cited Date:
- 2023-12-08
- Product:
- Journal:
- Mediators of Inflammation
- Cited Date:
- 2021-07-23
- Product:
- Journal:
- Scientific Reports
- Author:
- Department of Biochemistry and Molecular Biology, Faculty of Medicine, Universitas Indonesia, Jakarta, 10430, Indonesia
- Cited Date:
- 2024-01-12
- Product:
- Journal:
- Molecules
- Cited Date:
- 2022-01-07
- Product:
- Journal:
- Neurochemical Research
- Author:
- Department of Anesthesiology, The First Affiliated Hospital of Guangxi Medical University, Nanning, 530021, China.
- Sample:
- serum and hippocampus
- Cited Date:
- 2024-03-22
- Product:
- Journal:
- Journal of Ethnopharmacology
- Cited Date:
- 2021-08-26
- Product:
- Journal:
- Journal of Ethnopharmacology
- Cited Date:
- 2022-03-24
- Product:
- Journal:
- Journal of Cellular and Molecular Medicine
- Cited Date:
- 2019-05-29
- Product:
- Journal:
- Scientific Reports
- Cited Date:
- 2022-06-30
- Product:
- Journal:
- Green Processing and Synthesis
- Cited Date:
- 2023-03-17
- Product:
- Journal:
- Archives of Medical Science
- Author:
- Department of Rehabilitation Medicine, The Second Affiliated Hospital of Xi’an Medical University, Xi’an, Shaanxi, China
- Cited Date:
- 2023-08-18
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat IL-17(Interleukin-17) ELISA Kit
- Rat IL-2(Interleukin-2) ELISA Kit
- Rat Gfap(Glial fibrillary acidic protein) ELISA Kit
- Rat Th(Tyrosine Hydroxylase) ELISA Kit
- Rat SNCa(Synuclein Alpha) ELISA Kit
- Rat CXCR2(C-X-C chemokine receptor type 2) ELISA Kit
- Journal:
- Nutrition & Metabolism
- Cited Date:
- 2019-02-27
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat Nfe2l2(Nuclear Factor, Erythroid Derived 2 Like Protein 2) ELISA Kit
- Rat MCP-1(Monocyte Chemotactic Protein 1) ELISA Kit
- Rat MAPK(P38 Mitogen-Activated Protein Kinase) ELISA Kit
- Rat NF-κB p65(Nuclear Factor-κB P65) ELISA Kit
- Journal:
- hypertension research
- Cited Date:
- 2019-03-12
- Product:
- Journal:
- Molecules
- Cited Date:
- 2021-01-26
- Product:
- Journal:
- Food & Function
- Cited Date:
- 2020-07-30
- Product:
- Journal:
- Molecular Pain
- Cited Date:
- 2019-02-05
- Product:
- Journal:
- Molecular Pain
- Cited Date:
- 2019-05-30
- Product:
- Journal:
- Current Alzheimer Research
- Cited Date:
- 2020-09-05
- Product:
- Journal:
- Human and Experimental Toxicology
- Cited Date:
- 2021-07-09
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat TGF-β1(Transforming Growth Factor Beta 1) ELISA Kit
- Rat IL-10(Interleukin-10) ELISA Kit
- Rat MMP-9(Matrix Metalloproteinase-9) ELISA Kit
- Rat IFN-γ(Interferon γ) ELISA Kit
- Rat IL-21(Interleukin-21) ELISA Kit
- Rat CXCR4(C-X-C motif receptor 4) ELISA Kit
- Rat MMP-2(Matrix Metalloproteinase 2) ELISA Kit
- Rat TIMP-1(Tissue Inhibitors of Metalloproteinase 1) ELISA Kit
- Rat TIMP-2(Tissue Inhibitors of Metalloproteinase 2) ELISA Kit
- Journal:
- Brain Research
- Author:
- Obstetrics Department, Maternal and Child Health Hospital of Hubei Province, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, Hubei 430070, P.R. China
- Cited Date:
- 2023-09-01
- Product:
- Journal:
- Cardiovascular Toxicology
- Cited Date:
- 2023-04-14
- Product:
- Journal:
- Journal of Food Biochemistry
- Cited Date:
- 2021-12-30
- Product:
- Journal:
- Current Pharmaceutical Design
- Cited Date:
- 2019-11-19
- Product:
- Journal:
- Respiratory Physiology & Neurobiology
- Author:
- Biomedical Centre Martin, Jessenius Faculty of Medicine in Martin, Comenius University in Bratislava, Mala hora 4C, 036 01 Martin, Slovakia
- Cited Date:
- 2023-08-18
- Product:
- Journal:
- Pulmonary Circulation
- Cited Date:
- 2019-08-02
- Product:
- Journal:
- Pulmonary Circulation
- Cited Date:
- 2020-04-10
- Product:
- Journal:
- International Journal of Immunopathology and Pharmacology
- Cited Date:
- 2020-10-16
- Product:
- Journal:
- Pulmonary Circulation
- Cited Date:
- 2019-06-25
- Product:
- Journal:
- Archives of Physiology and Biochemistry
- Cited Date:
- 2020-05-16
- Product:
- Journal:
- European Journal of Trauma and Emergency Surgery
- Author:
- Department of Chemistry and Chemical Processing Technologies, Macka Vocational School, Karadeniz Technical University, 61750, Trabzon, Turkey
- Cited Date:
- 2023-07-14
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Rat Cat(Catalase) ELISA Kit
- Rat 8-OHdG(8-Hydroxydeoxyguanosine) ELISA Kit
- Rat MPO(Myeloperoxidase) ELISA Kit
- Rat Casp3(Caspase 3) ELISA Kit
- Rat ATF6(Activating transcription factor 6) ELISA Kit
- Rat Ddit3(DNA damage-inducible transcript 3 protein) ELISA Kit
- Rat Hmgb1(High mobility group protein B1) ELISA Kit
- Rat NF-κB p65(Nuclear Factor-κB P65) ELISA Kit
- Rat Hspa5(78 kDa glucose-regulated protein) ELISA Kit
- Journal:
- Evidence-Based Complementary and Alternative Medicine
- Cited Date:
- 2018-07-15
- Product:
- Journal:
- Medical Science Monitor
- Cited Date:
- 2020-01-26
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat IL-18(Interleukin-18) ELISA Kit
- Rat IL-17(Interleukin-17) ELISA Kit
- Rat Lipocalin-2/NGAL(Neutrophil Gelatinase Associated Lipocalin) ELISA Kit
- Rat IL-2(Interleukin-2) ELISA Kit
- Rat Cys-C(Cystatin C) ELISA Kit
- Rat Kim-1(Kidney Injury Molecule 1) ELISA Kit
- Rat NAGase(N-Acetyl Beta-D-Glucosaminidase) ELISA Kit
- Rat BMG/β2-MG(Beta-2-Microglobulin) ELISA Kit
- Journal:
- Food Science & Nutrition
- Cited Date:
- 2021-07-02
- Product:
- Journal:
- PHYSIOLOGICAL RESEARCH
- Cited Date:
- 2019-10-20
- Product:
- Journal:
- Neuroimmunomodulation
- Cited Date:
- 2021-05-07
- Product:
- Journal:
- Biotechnology and Applied Biochemistry
- Cited Date:
- 2020-10-30
- Product:
- Journal:
- Pharmacology
- Cited Date:
- 2019-08-30
- Product:
- Journal:
- Cellular and Molecular Biology
- Author:
- Department of Traumatic Surgery& Emergency Surgery, The 1st Affiliated Hospital of Wenzhou Medical University, Wenzhou, Zhejiang,325000, China
- Cited Date:
- 2023-10-13
- Product:
- Journal:
- High Altitude Medicine & Biology
- Cited Date:
- 2020-12-10
- Product:
-
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat TGF-β1(Transforming Growth Factor Beta 1) ELISA Kit
- Rat IL-17(Interleukin-17) ELISA Kit
- Rat IL-4(Interleukin-4) ELISA Kit
- Rat MCP-1(Monocyte Chemotactic Protein 1) ELISA Kit
- Rat SDF-1α(Stromal Cell Derived Factor 1α) ELISA Kit
- Rat IP-10/CXCL10(Interferon Gamma Induced Protein 10kDa) ELISA Kit
- Rat IL-23(Interleukin 23) ELISA Kit
- Rat IL-12(Interleukin 12) ELISA Kit
- Rat BLC(B-Lymphocyte Chemoattractant) ELISA Kit
- Journal:
- Journal of Nanoscience and Nanotechnology
- Cited Date:
- 2019-04-20
- Product:
- Journal:
- Brazilian Journal of Biology
- Cited Date:
- 2020-09-16
- Product:
- Journal:
- Turkish Journal of Trauma and Emergency Surgery
- Cited Date:
- 2023-02-10
- Product:
- Journal:
- Acta Poloniae Pharmaceutica - Drug Research
- Cited Date:
- 2020-03-13
- Product:
- Journal:
- Toxicology Reports
- Cited Date:
- 2019-05-21
- Product:
- Journal:
- Journal of Taibah University Medical Sciences
- Cited Date:
- 2019-07-23
- Product:
- Journal:
- European Journal of Inflammation
- Cited Date:
- 2018-05-14
- Product:
- Journal:
- Open Life Sciences
- Cited Date:
- 2021-02-26
- Product:
- Journal:
- Journal of Food and Nutrition Research
- Cited Date:
- 2018-12-19
- Product:
- Journal:
- Tropical Journal of Pharmaceutical Research
- Cited Date:
- 2021-10-08
- Product:
- Journal:
- Tropical Journal of Pharmaceutical Research
- Cited Date:
- 2022-06-17
- Product:
- Journal:
- Indian Journal of Animal Research
- Cited Date:
- 2022-09-02
- Product:
- Journal:
- Comparative Clinical Pathology
- Cited Date:
- 2019-06-25
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat BDNF(Brain Derived Neurotrophic Factor) ELISA Kit
- Rat BCL-2(B-Cell Leukemia/Lymphoma 2) ELISA Kit
- Rat Bax(Apoptosis regulator BAX) ELISA Kit
- Rat pτ(phospho Tau Protein) ELISA Kit
- Rat Aβ40(Amyloid Beta 40) ELISA Kit
- Rat CASP9(Caspase 9) ELISA Kit
- Journal:
- Anesthesia and Pain Medicine
- Cited Date:
- 2020-06-30
- Product:
- Journal:
- Research in Pharmaceutical Sciences
- Cited Date:
- 2022-09-08
- Product:
- Journal:
- United States Patent Application
- Cited Date:
- 2020-07-16
- Product:
- Journal:
- Farabi Medical Journal
- Cited Date:
- 2023-01-29
- Product:
- Journal:
- Biointerface Research in Applied Chemistry
- Cited Date:
- 2023-02-09
- Product:
- Journal:
- Novosti Khirurgii
- Author:
- Department of Surgery and Transplantation, Belarusian State Medical University, Minsk, Dzerzhinsky Ave. 83, 220116, Republic of Belarus
- Cited Date:
- 2023-10-08
- Product:
- Journal:
- Environmental Analysis Health and Toxicology
- Author:
- Department of Human Anatomy, Kaduna State University, State, Nigeria
- Cited Date:
- 2024-01-19
- Product:
- Journal:
- Farabi Medical Journal
- Author:
- Karadeniz Technical University, Faculty of Health Sciences, Department of Nutrition and Dietetics, 61080 Trabzon, Türkiye
- Cited Date:
- 2023-06-09
- Product:
- Journal:
- Clinical and Experimental Health Sciences
- Author:
- KARADENIZ TECHNICAL UNIVERSITY
- Cited Date:
- 2023-10-20
- Product:
- Journal:
- Research Square
- Cited Date:
- 2022-05-05
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Rat MDA(Malonaldehyde) ELISA Kit
- Rat TGF-β1(Transforming Growth Factor Beta 1) ELISA Kit
- Rat PTGS2/COX-2(Prostaglandin G/H synthase 2) ELISA Kit
- Rat Gsr(Glutathione reductase) ELISA Kit
- Journal:
- AIP Conference Proceedings
- Cited Date:
- 2020-09-16
- Product:
- Journal:
- research square
- Cited Date:
- 2020-09-29
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat PGE2(Prostaglandin E2) ELISA Kit
- Rat IL-10(Interleukin-10) ELISA Kit
- Rat IL-17(Interleukin-17) ELISA Kit
- Rat IL-13(Interleukin-13) ELISA Kit
- Rat 8-iso-PGF2α(8-isoprostane) ELISA Kit
- Rat CYSLTR2(Cysteinyl Leukotriene Receptor 2) ELISA Kit
- Mouse Ltb4r(Leukotriene B4 receptor 1) ELISA Kit
- Rat OVA sIgE(Ovalbumin specific Immunoglobulin E) ELISA Kit
- Journal:
- Research Square
- Author:
- Islamic Azad University
- Cited Date:
- 2024-02-18
- Product:
- Journal:
- Farabi Medical Journal
- Cited Date:
- 2023-04-07
- Product:
- Journal:
- Medicinski Glasnik
- Cited Date:
- 2021-07-15
- Product:
- Journal:
- Sahel Journal of Life Sciences FUDMA
- Author:
- Department of Biochemistry and Molecular Biology, Federal University Dutsin-Ma, P.M.B 5001, Katsina State, Nigeria
- Sample:
- serum
- Cited Date:
- 2024-03-08
- Product:
- Journal:
- Journal of Nutrition and Health
- Cited Date:
- 2020-03-13
- Product:
- Journal:
- Comparative Clinical Pathology
- Cited Date:
- 2019-02-15
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- 8-OHdG(8-Hydroxydeoxyguanosine) ELISA Kit
- Rat Cat(Catalase) ELISA Kit
- Rat Gpx1(Glutathione peroxidase 1) ELISA Kit
- MDA(Malondialdehyde) ELISA Kit
- Journal:
- F?rat University Medical Journal of Health Sciences
- Author:
- Gaziantep University, Faculty of Medicine, Department of Physiology, Gaziantep, T?RK?YE
- Cited Date:
- 2023-07-07
- Product:
- Journal:
- F1000Research
- Cited Date:
- 2022-12-16
- Product:
- Journal:
- bioRxiv
- Author:
- Department of Medical Laboratory Science, Faculty of Health Sciences and Technology, Nnamdi Azikiwe University, Nnewi, Nigeria
- Cited Date:
- 2023-09-15
- Product:
- Journal:
- Journal of International Dental and Medical Research
- Cited Date:
- 2020-05-02
- Product:
- Journal:
- Research Square
- Cited Date:
- 2022-03-10
- Product:
- Journal:
- HEPATITIS MONTHLY
- Cited Date:
- 2020-05-10
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat IL-1β(Interleukin 1 Beta) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- Rat MDA(Malonaldehyde) ELISA Kit
- Rat Cat(Catalase) ELISA Kit
- Rat 8-OHdG(8-Hydroxydeoxyguanosine) ELISA Kit
- Rat Gpx1(Glutathione peroxidase 1) ELISA Kit
- Rat BCL-2(B-Cell Leukemia/Lymphoma 2) ELISA Kit
- Rat Bax(Apoptosis regulator BAX) ELISA Kit
- Rat CASP9(Caspase 9) ELISA Kit
- Journal:
- Beni-Suef University Journal of Basic and Applied Sciences
- Cited Date:
- 2023-01-06
- Product:
-
- Rat TNF-α(Tumor Necrosis Factor Alpha) ELISA Kit
- Rat IL-6(Interleukin-6) ELISA Kit
- Rat SOD1(Superoxide dismutase [Cu-Zn]) ELISA Kit
- 8-OHdG(8-Hydroxydeoxyguanosine) ELISA Kit
- Rat Cat(Catalase) ELISA Kit
- Rat MPO(Myeloperoxidase) ELISA Kit
- Rat Casp3(Caspase 3) ELISA Kit
- Rat ATF6(Activating transcription factor 6) ELISA Kit
- Rat Ddit3(DNA damage-inducible transcript 3 protein) ELISA Kit
- Rat Hmgb1(High mobility group protein B1) ELISA Kit
- Rat NF-κB p65(Nuclear Factor-κB P65) ELISA Kit
- Rat Hspa5(78 kDa glucose-regulated protein) ELISA Kit